How to develop and implement a quality management system (QMS)
Any new and forward-looking business, particularly one in a regulated industry, will ultimately face the challenge of how to implement a quality management system.
In fact, knowing how to set up and how to develop a quality management system isn't just one more task on your 'to do' list, next to hiring staff and securing funding. It should be your #1 priority.
Why? The quality management system (QMS) is the beating heart of your organization, and the nerve center holding everything together. Taking the time to learn how to build a quality management system, and to ensure it works, is the difference between success and failure in the critical early days of your business.
Fortunately, you're in the right place. Here's how to build a QMS system and lift your business to the next level.
How to develop a quality management system
Where to begin?
There's no getting around the fact that QMS implementation is a complex, long-term task. It can be tricky knowing where to even start.
Developing the initial framework of what you want your QMS to be is always a sensible first step.
And, as the generic quality management standard, ISO 9001 is the best documented regulation to align your initial QMS building work with.
Here's a handy QMS 'map' to help you visualize the interacting parts you'll need to get in place.
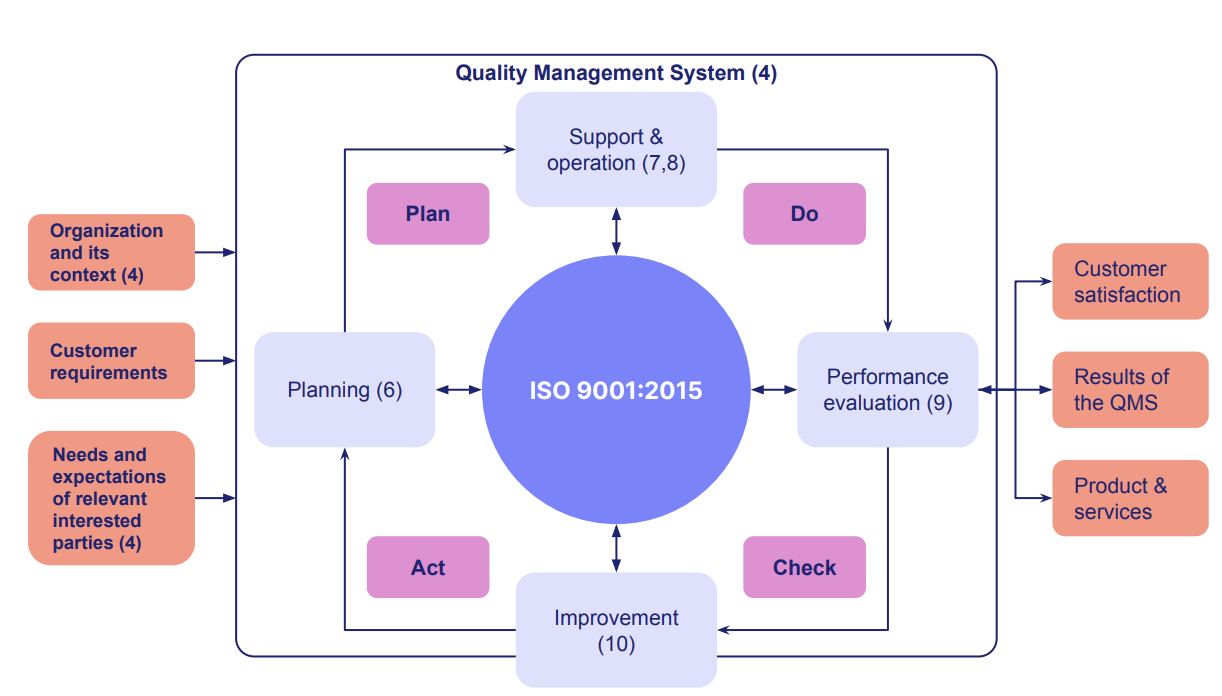
There's a lot to unpack here, so let's simplify the process down to 5 key steps - 2 in the development stage, 3 in the implementation stage.
Step 1: The stakeholder(s)
Understanding stakeholder needs and requirements is the fundamental core of how to create a QMS.
After all, quality is defined by ISO as:
"... the degree to which a set of inherent characteristics of an object fulfils requirements."
To embed quality, you need to meet the requirements of your stakeholders: most importantly, your customers and (for life science companies) your patients and your regulators.
And to meet those requirements, you need to know what they are.
What product and service will you provide, and within what parameters?
Determine the boundaries and applicability of your QMS. This involves identifying the processes, products, services and locations that the QMS will cover.
Define the context of your business with a SWOT and PESTLE analysis, then identify and review the internal and external factors that will impact the business, including who your stakeholders (or, as ISO 9001 defines them, 'interested parties') are.
Conduct market research, gather feedback from existing customers if you have them, and review the regulations that will be relevant to your sector and to your short- and long-term market plans.
Define who and what is relevant to the QMS, then use this information to set its scope and boundaries.
Once you've done that, consider how you'll continuously review and monitor these parameters to ensure they're still relevant and applicable to you.
This information will form the basis for how to start a quality management system in your business, while ensuring it addresses all necessary compliance and stakeholder criteria.
%20.png?width=1474&height=168&name=How%20to%20Start%20%26%20Build%20a%20Quality%20Management%20System%20(QMS)%20.png)
Step 2: Top-down buy-in
A quality management system can't be run in the long term without securing commitment from your top management.
Leadership plays a crucial role in establishing a quality-focused culture and providing the necessary resources for the QMS.
This includes defining the quality policy and objectives that align with the organization's strategic goals.
As such, clearly organize and structure your operational hierarchy with clear roles and responsibilities. Ensure frequent review touchpoints are in place for leadership to review the QMS and demonstrate their commitment to quality.
Above all, ensure customer, statutory and regulatory requirements are understood, systematized and met by senior leadership - who, in turn, should then constantly communicate and enforce the quality policy and your QMS requirements business-wide.
Remember: it is top management’s responsibility to ensure your critical QMS tasks are planned, implemented and achieved. Be on your guard against a siloed quality person or department, and push for C-level involvement from the earliest days of your QMS build.
Make sure your leadership team:
- Inform everyone of the importance and benefits of an effective QMS
- Tell everyone why they should participate in its effective implementation
- Ensure the quality policy and objectives are compatible with the strategic direction and context of your organization
- Promote risk-based thinking (more on that below) in respect of your organization’s quality management system
- Make sure the management system achieves its intended outcome
- Ensure there are adequate resources to maintain the quality management system
- Ensure the effectiveness of the quality management system
How to implement a quality management system
Now we've covered the initial phase of how to start a quality management system, it's time to move onto how to create a quality management system and bring it into being with an end-to-end implementation.
Step 3: Building processes
Thoroughly documented, consistent and understood processes are the lifeblood of how to implement a quality management system.
And your processes should at all times be built with risk, and risk-based thinking, as a guiding light.
Establish processes for systematically managing risk, embedding risk-based thinking and following a risk-based approach.
In short, this means determining, considering, and taking action to address any risks and opportunities that could impact your organization’s ability to deliver the intended results and 'requirements' you identified in Step 1.
External risks could be:
- Government
- Authorities
- Regulators
- Customers
- Trade bodies
- Staff dependents
- Competitors
- Suppliers
- Business owners
- Bankers/investors
- Business partners
- Contractors
Internal risks could include:
- Contractors
- Business partners
- Management
- Quality & compliance
- Health & safety
- Risk management teams
- Business development
- Marketing
- HR
- Finance
- Purchasing
- Facilities & estates
- Manufacturing
- Procurement
Follow this process to ensure risks are properly documented, assigned and treated:

Once you've done that, it's time to set the quality objectives for every function and department within the QMS.
These end goals, taking into account your stakeholder requirements, will define how you start to build and execute your day-to-day business processes.

How to create a QMS bundle of processes and procedures?
Start with the 'core' activities that are critical to what your business does.
Ask yourself which processes play a significant role in ensuring:
1) You are constantly providing products and services which meet customer, statutory and regulatory requirements
2) You are enhancing customer satisfaction
Some critical QMS processes to prioritize are:
1. Manufacturing
2. Change management
3. Risk management
4. Incident management
5. Documentation lifecycle management
6. Customer/supplier onboarding
7. Internal auditing
8. Quality control/assurance
Then think about how those processes will relate to, and interact with, each other.
Here's an example from our own quality manual to give you some inspiration:

Step 4: Supporting processes
Now the processes are built, the next phase of how to create a quality management system rests on bringing those processes to life and 'supporting' them with that they need to work.
That means finalizing your processes for controlling the creation and updating of information streams, including documentation, and ensuring employee competency with a training program.
The aim here is to ensure that competence and communication are baked into how your processes operate day-to-day, so everyone has access to the information and training they need to do their jobs in a consistent and compliant way.
Think about the inputs, outputs, resources and controls each process needs, and how you'll start delivering your outputted products and services to customers.
Read the 5 things every life science QMS needs for strong processes
Step 5: Measuring & improving
Continuous improvement should be at the heart of how you build your quality management system.
That means ensuring you can consistently and accurate measure how your processes perform, so that you can, in turn, seek and act on improvement opportunities.
There are two main ways you can continuously take the temperature of your quality management system:
1) With internal audits
2) With frequent management reviews
Knowing how to audit the process-based QMS is important too, so here are some typical quality management KPIs you could choose to measure:
- % of processes where completion falls within +/- 5% of the estimated completion
- Sum of costs of 'killed'/stopped active processes
- Customer happiness (NPS)
- Average process overdue time and % of overdue processes
- % of processes where assigned resources exceeds planned number
- Average time to complete tasks
- Defect and nonconformance rates
One of the main benefits of a quality management system is arming yourself with this information, then harnessing it to continuously improve.
Any issues and weaknesses should be targeted and fixed with CAPA processes, while also ensuring more proactive, improvement-based opportunities are acted on where possible.
The 'As-Is' model is a good way to apply improvement-based thinking into your QMS:

It's important to note here, too, that recent industry initiatives like the FDA's Quality Management Maturity program for pharmaceutical businesses encourage your quality management system to be as proactive as possible - minimizing 'firefighting' so maximum time can be spent on planned improvement activity.

To do this, you'll need to automate time-consuming QMS admin tasks like document control and manual training, and free up as much time as possible for the quality department to be proactive and future-focused. eQMS software is a great way to achieve this.
FURTHER READING: Total quality management (TQM): definition and principles
Process map: how to implement quality management system
To help you move forward with a more detailed, step-by-step action plan, here's a handy process map you can apply to your own operation:

Remember to use ISO 9001 as the basic framework for your QMS, before layering on additional industry-specific regulatory requirements.
How to implement a quality management system with ease
As you've probably learned by now, answering the question "how to develop a quality management system" isn't simple.
More and more quality-centric businesses are turning to providers of digital tools, like Qualio, to automate and accelerate their critical QMS tasks.
QMS software doesn't only help you develop and implement a quality management system at speed. A single, cloud-based source of truth connects your entire business, including senior leadership, to the quality agenda and encourages business-wide contribution and support.
Configurable digital workflows standardize your processes, while analytic dashboards give you the insights you need to spot weaknesses and drive continuous improvement.
Plus, pre-built QMS document templates specific to your industry regulations provide instant shape and structure to your quality system, and get your entire company on the same page.
Just check out the difference an eQMS makes, based on our recent 2024 life science quality survey:


Every company ultimately needs to learn how to implement a quality management system. But the tools you use to manage it matter just as much as getting one in place. Plan carefully, choose wisely - and the benefits will be dramatic.
