What to know about document control software in 2025
Document control software is now an indispensable tool of modern life science quality management. Quality is impossible without structured, compliant information - and for that, document management version control and associated document version control tools are critical.
If your business is still using legacy tools like paper and spreadsheets to manage information, it's likely that document control software is a worthwhile investment for you. From slicing regulatory risk and embedding GDocP, to just saving time and getting all your colleagues literally on the same page, document control software should be part of how you do business in 2025.
With that in mind, let's explore what document control software is, and how to identify the best platform for your needs.
What is document control software?
Document control software is a type of software designed to manage, track and control documents within your organization. A document control software system provides a centralized platform for storing, organizing, accessing and distributing documents, ensuring that the right people have access to the right documents at the right time while maintaining security and - as the name suggests - compliant control.
Key features of document control software typically include:
-
Document storage and organization: The software allows users to store documents in a centralized repository, organized in a hierarchical structure or tagged with metadata for easy search and retrieval.
-
Version control: Document control software tracks changes to documents over time, allowing users to access previous versions, compare changes, and revert to earlier versions if necessary. Change control software like this ensures documents are updated with integrity and control.
-
Access control and permissions: Administrators can set access permissions to control who can view, edit, delete and share documents, ensuring that sensitive information is only accessible to authorized personnel.
-
Collaboration tools: Some document control software systems, like Qualio, even include collaboration features like commenting, annotation, and real-time editing, enabling teams to work together on documents regardless of physical location.
-
Workflow automation: The software streamlines document approval processes by automating workflows, routing documents to the appropriate stakeholders for review and approval based on predefined rules and deadlines.
-
Audit trails and compliance: Document control software maintains detailed audit trails that track all document-related activities, including views, edits, and approvals, to ensure compliance with regulatory requirements and internal policies.
Document control tools help organizations improve efficiency, reduce errors, enhance collaboration and ensure compliance with regulatory standards by effectively managing their document lifecycle from creation to archival.
Document version control tools
Why does your business need document control software?
Document version control tools are vital in highly regulated industries where operational errors, such as a worker following an outdated SOP on a medical device assembly line or in a pharma manufacturing process, can impact product quality and therefore patient safety.
Airtight version control is crucial for both your quality management system's integrity and for compliance with international ISO quality standards.
QMS document control software
Document control is an integral part of the modern quality management system. Strong document control procedures and processes should be built and distributed across your business to ensure information is properly maintained and people access the right information at the right time to do their jobs effectively.
So why can't you just use popular tools like Word for your document management version control, and save your company some money?
Unfortunately, generic unspecialized document systems like Word are great for building documents, but not for the associated control and integrity activities you'll need in any regulated industry which demands a quality management system (QMS).
For this reason, you'll sometimes see document control software referred to as 'QMS document control software' or 'quality document control software'.
QMS document control software is any platform which gives you the ability to control quality management documents like SOPs, work instructions, policies, quality manuals and so on, with the extra features and functionality you'll need for full document control.
A useful way to think about modern document control requirements is to consider the 'ALCOA++' data integrity principles originally developed in the pharmaceutical industry, and which are becoming an increasingly widespread benchmark for how documents are managed and controlled.
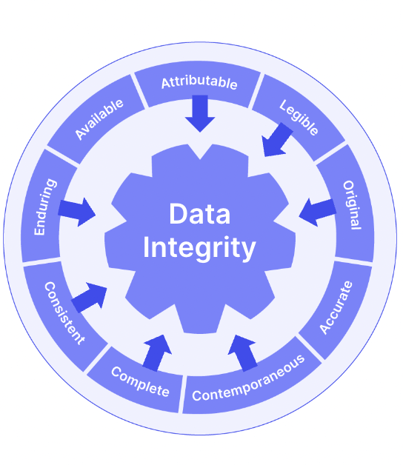
Only dedicated document control software systems contain the functionality and guardrails you'll need to ensure your documents meet quality and ALCOA++ requirements.
Hear one of our customers explain why you simply can't use Word for document control activity:
Learn more about how a document control system strengthens your quality
ISO document control software
Closely connected to this is the importance of applying document version control tools when thinking about compliance with ISO quality standards.
All modern ISO standards are built around the Annex SL high-level structure. The requirements in Clause 8, 'Operation', are difficult to meet without controlled, compliant and well-documented information streams, and it's for this reason that regulated companies considering ISO certification usually opt for document control software at the same time.
In particular, the ISO 27001 information security standard enforces strict processes and controls for documents and data. Document version control software is therefore crucial for compliance with this standard.
Above all, investing in a reputable document control software system is a great way to accelerate your route to ISO compliance and avoid the typical blunders and slip-ups that ISO auditors spot.
But it's worth noting that any document management version control tool investment should be underpinned by strong internal procedures, a quality-focused culture and aligned ways of working.
Document control software by itself can only get you some of the way to full QMS integrity and ISO compliance.
Top document control software
Now we've touched on what document control software does, how do you identify the best document control software for your business?
Over 650 life science companies trust and love Qualio, but we’re not the right fit for every organization. Global corporations have different document version control needs to scrappy young companies.
For example, if you’re a medical device start-up or scale-up transitioning from paper, we've built simplicity, configurability, ease of use, and scalability into our platform because those are the attributes you need in the early stages of your business. We're engineered to increase speed-to-market.
However, if you’re a global enterprise in the consumer goods vertical with 40,000 users in 20 countries, a solution that is extremely customizable and built for your industry is likely more of a determining factor in your selection process.
Finding the best document control software for your firm should involve narrowing your search to vendors who fit your industry, budget and size. In addition, look deeper than the marketing to learn who’s really at the top of their game. Real customer reviews can reveal a lot about satisfaction, ease of use and quality of support over the long term.
We laid out our list of the best document management software for life sciences in a separate article and encourage you to check that out, too!
ETQ
ETQ is a top eQMS option for enterprise and mid-sized businesses in multiple industries, including electronics, manufacturing and food. It could be among the best options if you’re looking for a solution with document management capabilities. It offers built-in best practices and the flexibility to customize your user experience and workflows.
ETQ document control software review
ETQ is an expensive monster application with 42 'applications', including document control, available to customers.
It's therefore best if you need a full 'bells and whistles' digital quality approach with layers of modular functionality requirements.
Customers on G2 report a fairly intuitive document control software interface, with helpful support for when problems do arise.
However, they also note high levels of customization needed to mold the system to internal requirements, with Python and SQL expertise needed.
Search functionality is reported as being slow. And most critically of all, ETQ has no native document editing functionality, forcing users to make edits in external tools like Google Docs before re-uploading into the platform.
This 'in and out' approach can weaken your document control and integrity if a clear process isn't established and followed.
Conclusion:
Good for large companies with large budgets, comfortable with large levels of manual customization.
Not good for editing, collaboration or quick, lean 'one-stop shop' access to your document control system.
QT9
QT9 is a web-based or self-hosted solution for highly regulated businesses of all sizes, including life science companies and aerospace firms. It offers a mind-boggling array of 23 modules and a broad suite of typical document control software capabilities, including categorization and FDA 21 CFR Part 11-compliant e-signatures.
QT9 document control review
Is QT9 the best document control software choice for you?
G2 customers report solid document control software functionality, including traceable change histories and audit trails to track document histories.
But the software scores less well from a user experience (UX) perspective, with users noting a cluttered and unclean interface with lots of invasive drop-down menus.
Like ETQ, QT9 offers no native document editing, forcing you to perform document building tasks in external third-party systems before returning them to the system for storage.
Plus, a concurrent license model can get in the way of robust document control by forcing users to share logins or wait for other users to exit the platform before being able to log in.
Conclusion:
Good for integrating with a separate ERP platform and for storing paperless documents with typical document control guardrails.
Bad for licensing, user experience and checking documents in and out for edits.
Greenlight Guru
Greenlight Guru typically comes head-to-head with Qualio when medical device start-ups and scale-ups look for a reputable document control software system. It's designed exclusively for medical device manufacturers, particularly hardware, and offers a fixed document control software environment for small companies to adopt.
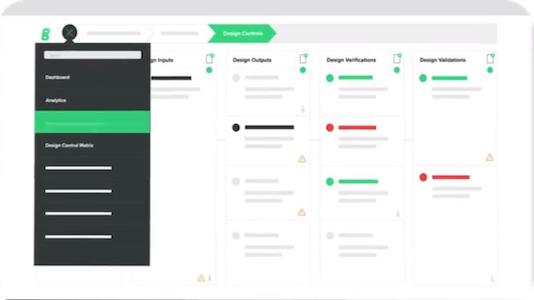
Greenlight Guru review
Like most document control software systems, Greenlight Guru offers typical functionality like cloud storage, traceability and e-signatures.
And its lean, focused structure means it scores highly with customers for usability and intuitiveness.
But for general business-wide document control, it may prove a more expensive investment than Qualio. All document users, even 'lite' users, are chargeable. Its more rigid set-up makes it more difficult to scale with you as your document needs evolve, and it too offers no native document editing as attachments are uploaded into the system from external sources.
Conclusion:
Good for start-ups and scale-ups that want a pre-built, inflexible and easy-to-use document control area to replace paper.
Bad for access costs, editing, scalability and configurability.
MasterControl
MasterControl is an industry-leading enterprise solution for the biggest highly regulated firms worldwide who can dedicate significant financial resources toward customized document control software technology. MasterControl's document control software must be purchased separately from other MasterControl products such as their QMS, Audit and Manufacturing modules.
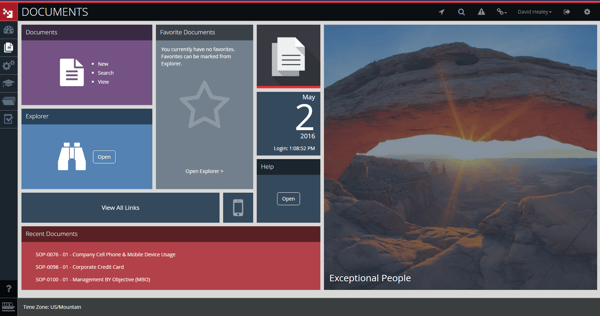
MasterControl document control review
MasterControl's G2 reviewers note difficulty accessing documents within the software due to long load times and clunky search functionality which requires exact title matches to display results.
Like Greenlight Guru, ETQ and QT9, its lack of native editing means document control activity must be split across multiple tools and systems, with the platform acting as a repository for documents edited elsewhere.
And despite its Windows-influenced UX, the software doesn't score well for general usability, with a 2-week starter course and module-specific subject matter experts required.
Conclusion:
Good for building a large enterprise quality management system that includes document control functionality among an array of other modules and add-ons.
Bad for native editing, usability and time to value.
Qualio
Qualio is the leading document control software platform for life science start-ups and scale-ups. As the first cloud eQMS built specifically around FDA and ISO best practice, Qualio offers the advantage of industry-leading time-to-value and off-the-shelf functionality.
Qualio was developed to offer exceptional document management version control and general document control capabilities such as rapid search, templating, and workflows.
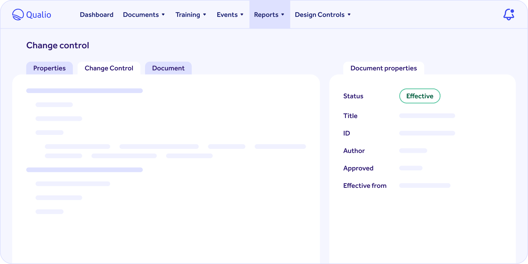
Qualio quality document control software review
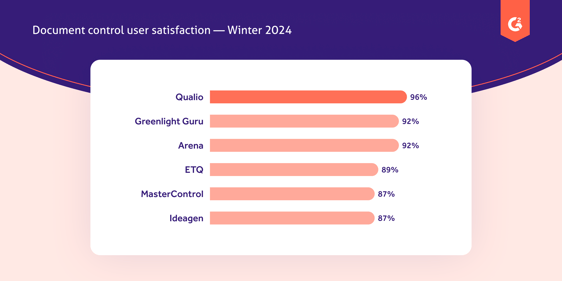
Qualio's document control software customers are the happiest of all, with a whopping 96% approval rate on G2.
They report a 90+% reduction in document control admin time, as well as a clean, user-friendly interface that minimizes clutter and maximizes internal engagement.
Qualio is built with a hybrid document control approach at the core, to make both control of existing documents and construction of new documents as easy and intuitive as possible.
A native OneDrive integration means document management version control can be extended to all your existing documents - even those stored and worked on by your colleagues in the company OneDrive folder they know and love. Flexible file format support allows instant upload into your eQMS without time-consuming conversions, accelerating your time to value.
And Qualio remains the only document control software system on the market with a fully native in-app document editor, allowing the system to be used as a one-stop shop for your entire document lifecycle from drafting and review of new documents to distribution and archiving.
Conclusion:
Good for rapid time-to-value, flexible file formats, scalability, configurability, integration, and for life science start-ups and scale-ups who need the most intuitive, easy-to-use document control software platform available - without having to check documents in and out of other systems for edits and updates.
Bad for enterprise operations who need complex functionality with dozens of adjoining modules.
Which is the best document control software?
Every document control software option that made our 2025 list is a trusted option.
However, user reviews aren’t the only thing to keep in mind. Be realistic about the resources you can dedicate to implementing and managing your document control software long-term, and avoid software that’s too heavy or too lightweight for your needs.
When you’ve narrowed your choices, the next best step is to start a conversation with vendors and schedule a demo. Your document version control software should bring advantages in efficiency, compliance, simplicity and transparency.
Start by booking your Qualio demo now: