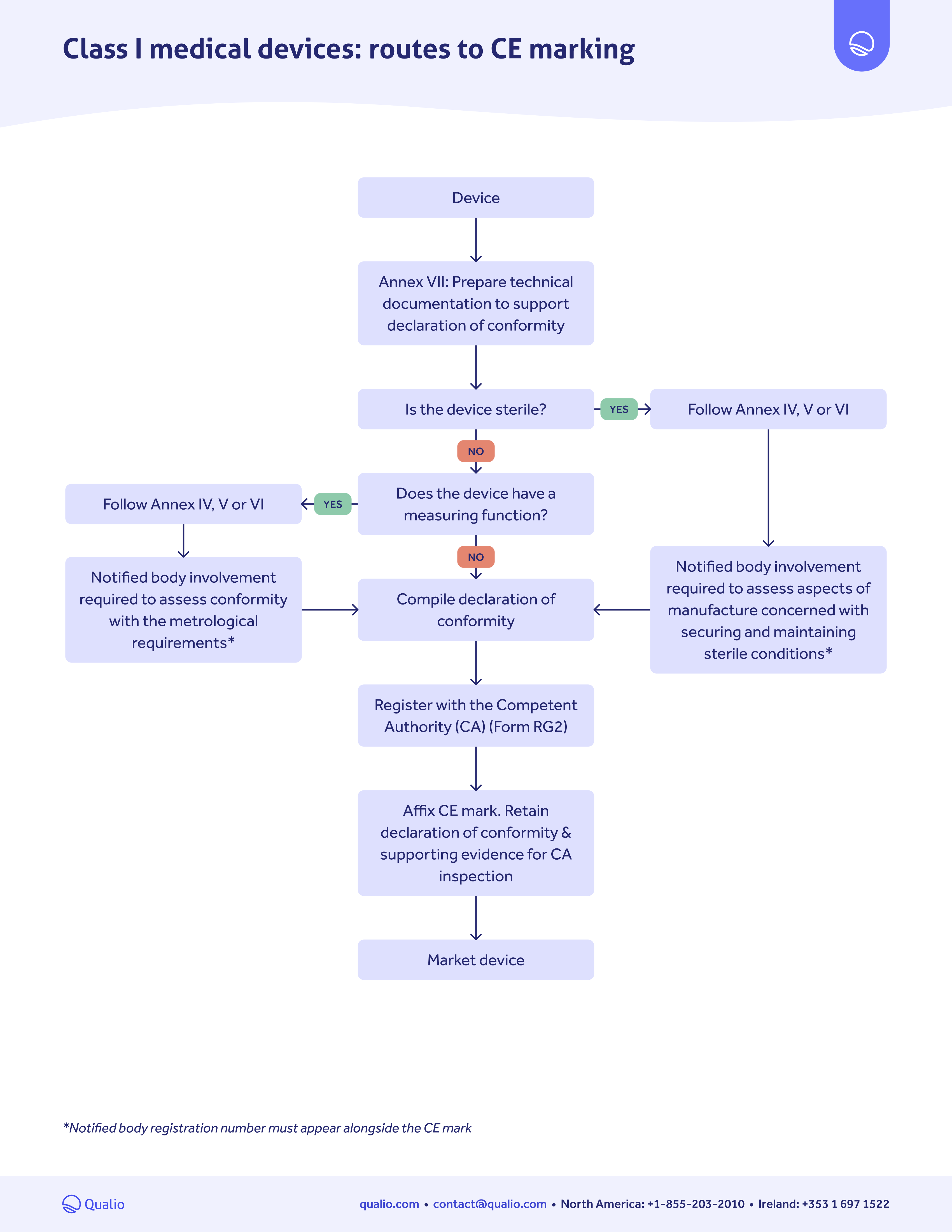
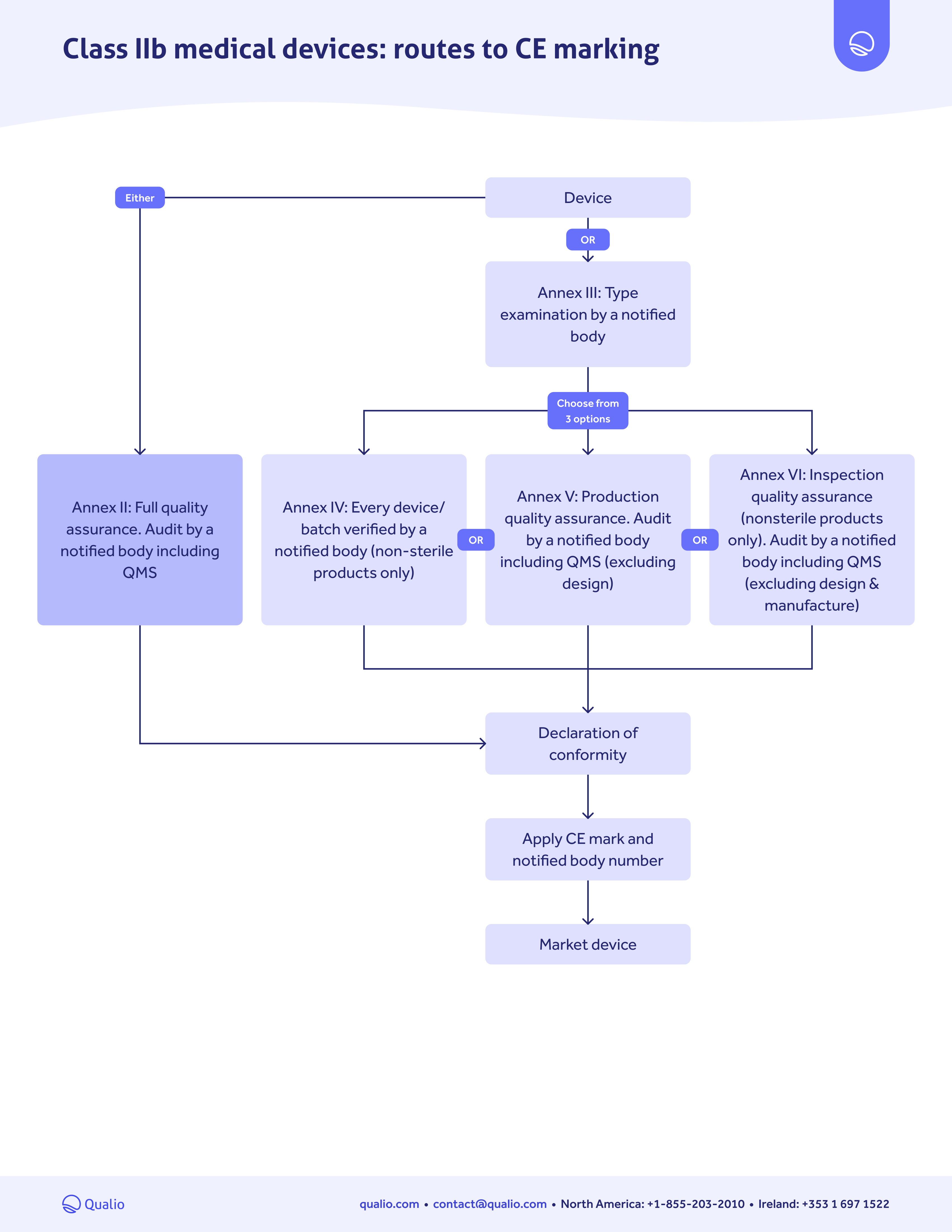
CE marking pathway guide
Get a step-by-step, class-specific pathway map to secure a CE mark for your medical device.
Instant access. No email required.
Download our CE marking medical device pathway guide to:
- Get a step-by-step action guide for getting your medical device approved for the EU market
- Follow the right pathway depending on your device risk class
- Drive a controlled, confident pathway to EU approval and CE marking
What you'll get:
Class-based map
Learn which actions you need to take for EU market approval, depending on the risk class of your medical device
Stress-free CE marking pathway
Step-by-step guidance