EU MDR software datasheet
Learn how Qualio customers use our software to meet the requirements of the EU MDR.
Download our EU MDR compliance software datasheet to learn how Qualio:
- Arms your business with a complete, EU-compliant eQMS for medical device quality management
- Supports and simplifies compliance with Regulation (EU) 2017/745, more commonly known as the Medical Device Regulation (MDR)
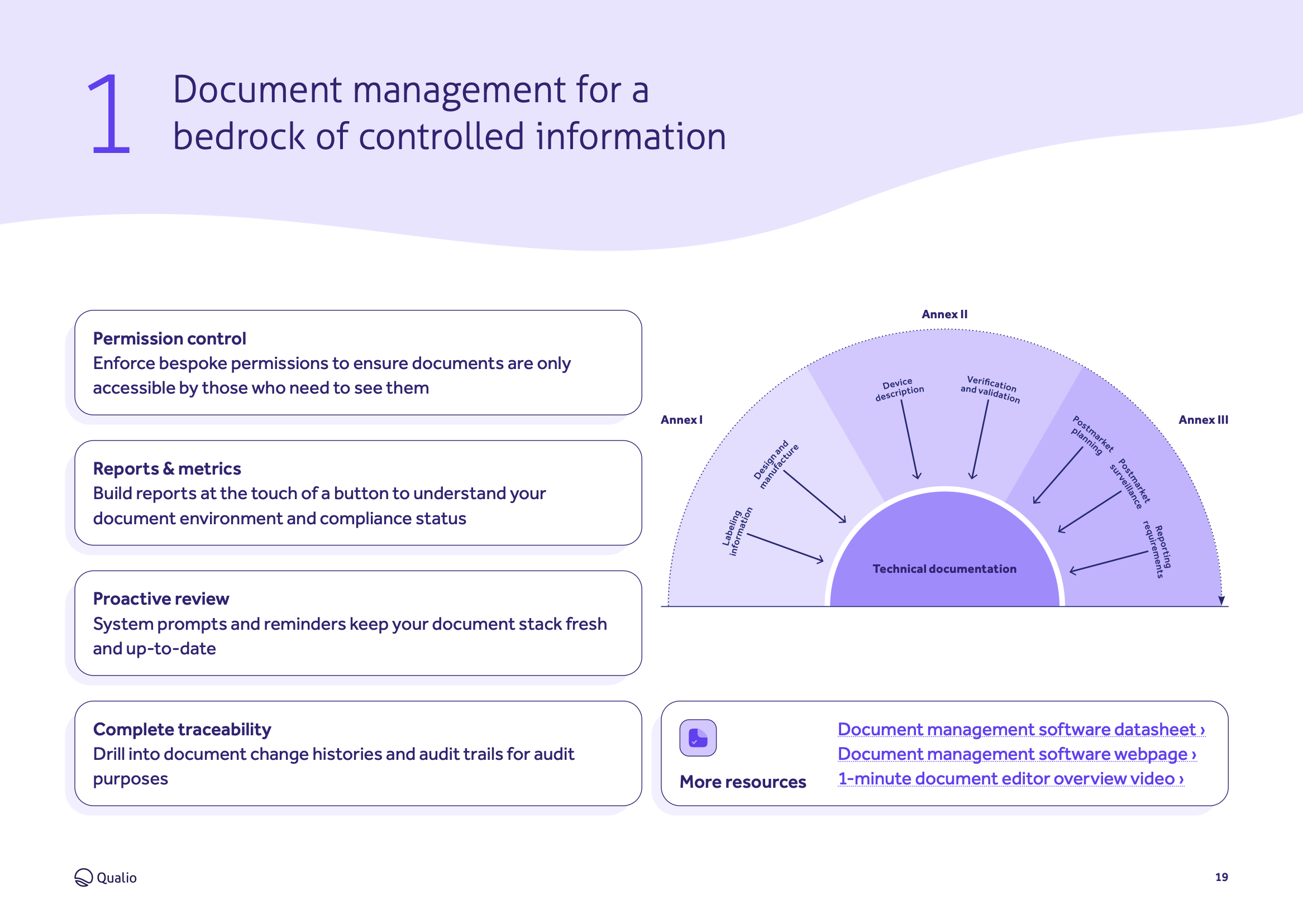
- Digitizes and automates all areas of medical device quality management, from design controls to document management
Complete the form to the right to get started!
What you'll get:
Feature breakdown
Explore the core features and functionality of Qualio, and how each area of the system contributes to a holistic medical device eQMS
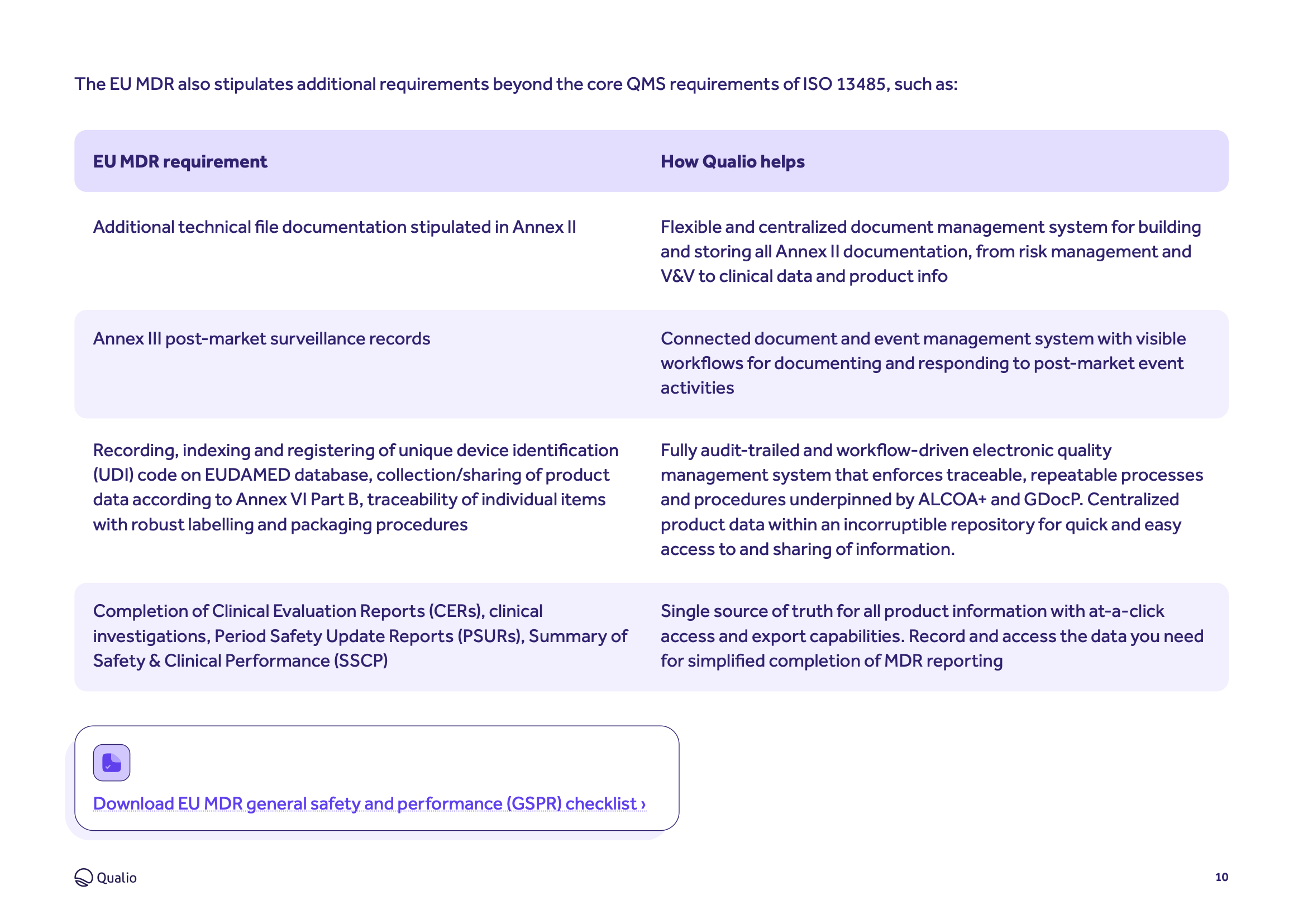
Complete EU MDR compliance
Learn how Qualio helps your business meet the demands of each section, annex and chapter of the Medical Device Regulation
Set-up, services and more
Hear from real Qualio medical device customers, learn how we onboard and implement our software, and access more helpful resources