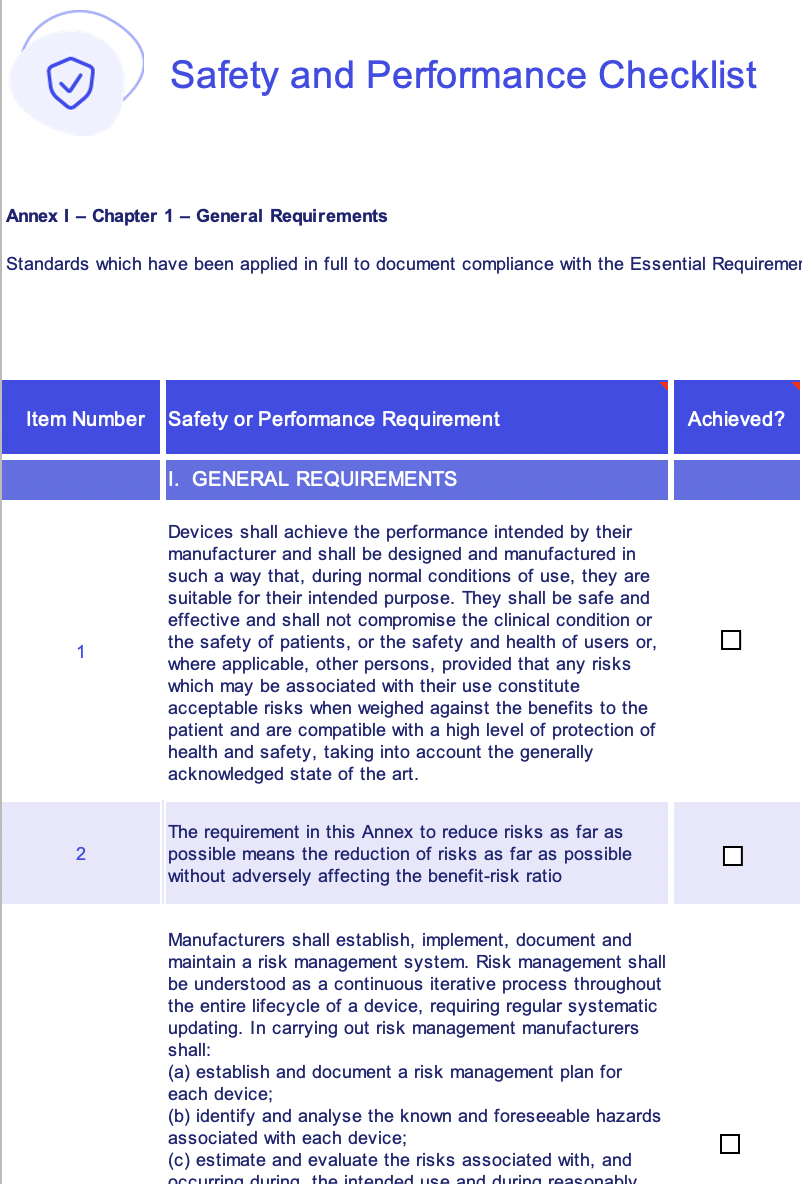
EU MDR general safety and performance requirements (GSPR) checklist
Meet the EU MDR's general safety and performance requirements for your medical device.
Download our EU MDR general safety and performance requirements (GSPR) checklist to:
- Understand every requirement your business and device(s) must follow for EU-compliant medical device safety and performance
- Take controlled step-by-step action to get compliant
- Pinpoint gaps in your medical device quality management system and satisfy your EU notified body inspector
Complete the form to the right to get started!
What you'll get:
4-part XLSX checklist
Access a comprehensive and ordered Excel checklist to work your way through your GSPR demands
Itemized action points
Annex-specific requirements list