ISO 13485 toolkit
Master and comply with the core quality management standard for medical device companies.
Instant access. No email required.
ISO 13485 is the definitive quality standard for organizations involved in the design, development and manufacture of medical devices.
Download our ISO 13485 toolkit to:
- Get to grips with the requirements of the standard and what you need to do to comply
- Start taking actionable steps towards robust and long-term ISO 13485 compliance
- Become completely audit-ready and bring a safe, high-quality device to market
What you'll get:
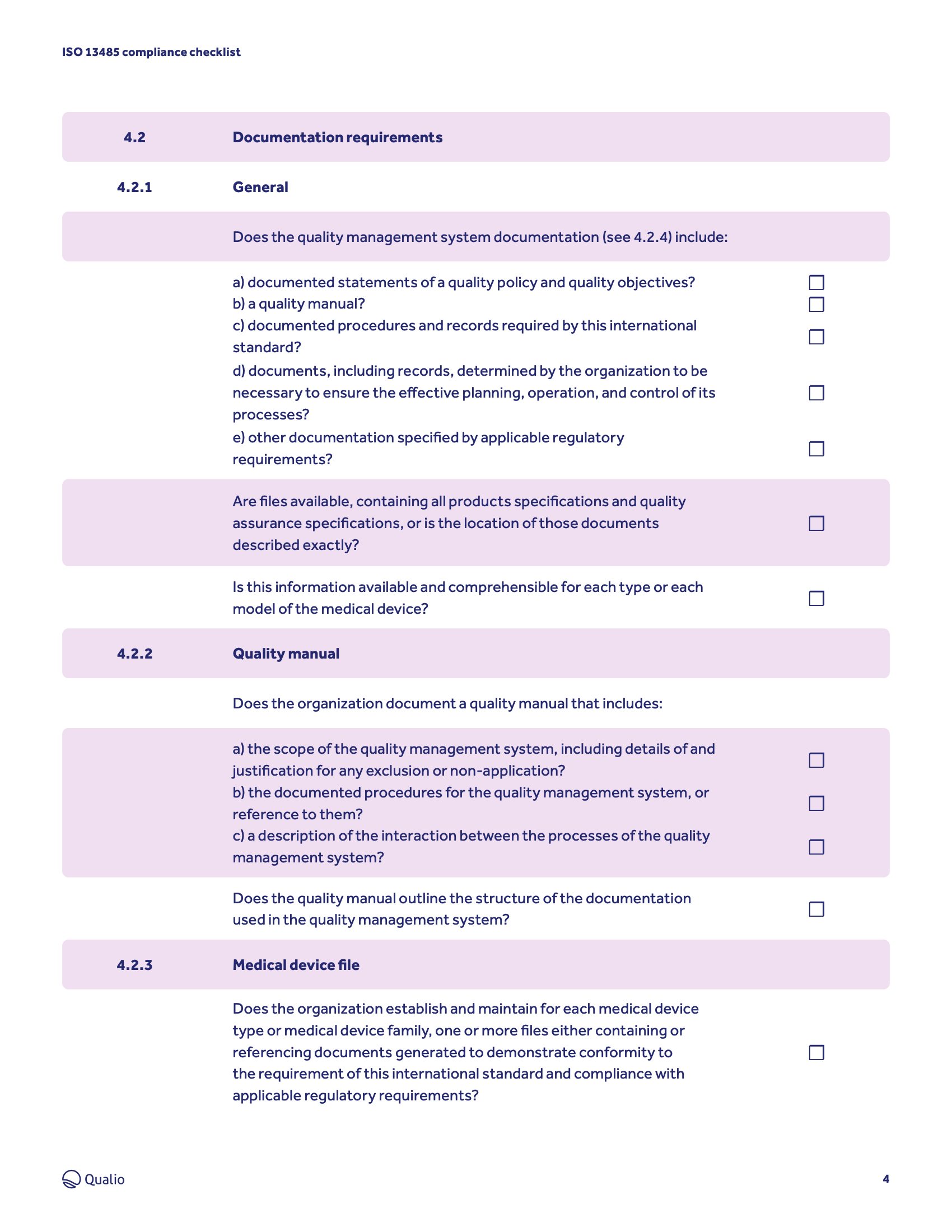
Compliance checklist
Preparing for ISO 13485 certification guide
Audit readiness checklist for medical device companies
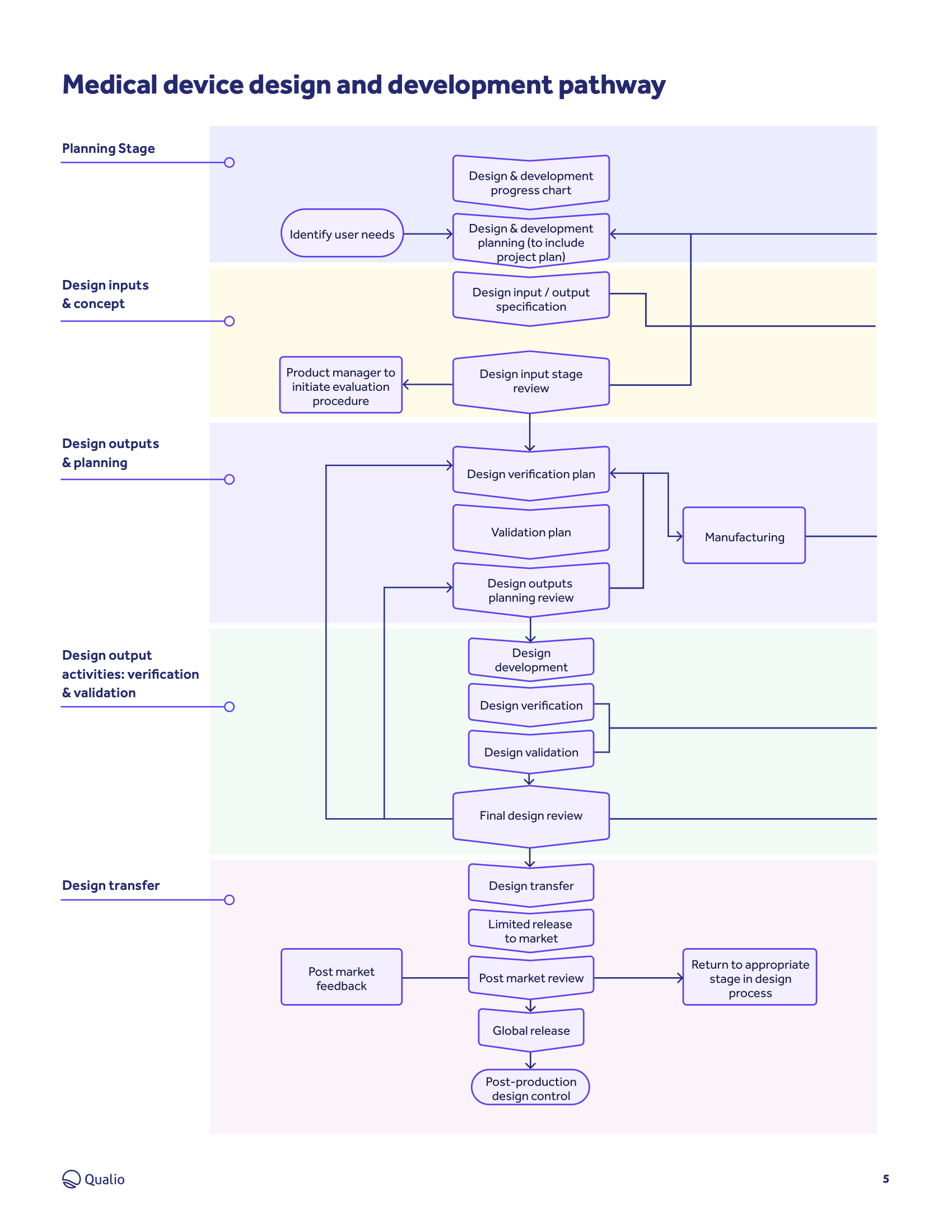
Ultimate guide to medical device design controls
Design controls: 6 principles for success
9 ways to improve product quality in medical device product development
Assembling a Design History File (DHF) for your medical device
Know your DHF from your DMR? Need a rundown of the key ingredients of a Design History File? Our walkthrough guide runs you through everything you need to know
Risk management plan template
Access a ready-made two-page document template for an ISO 13485 risk management plan
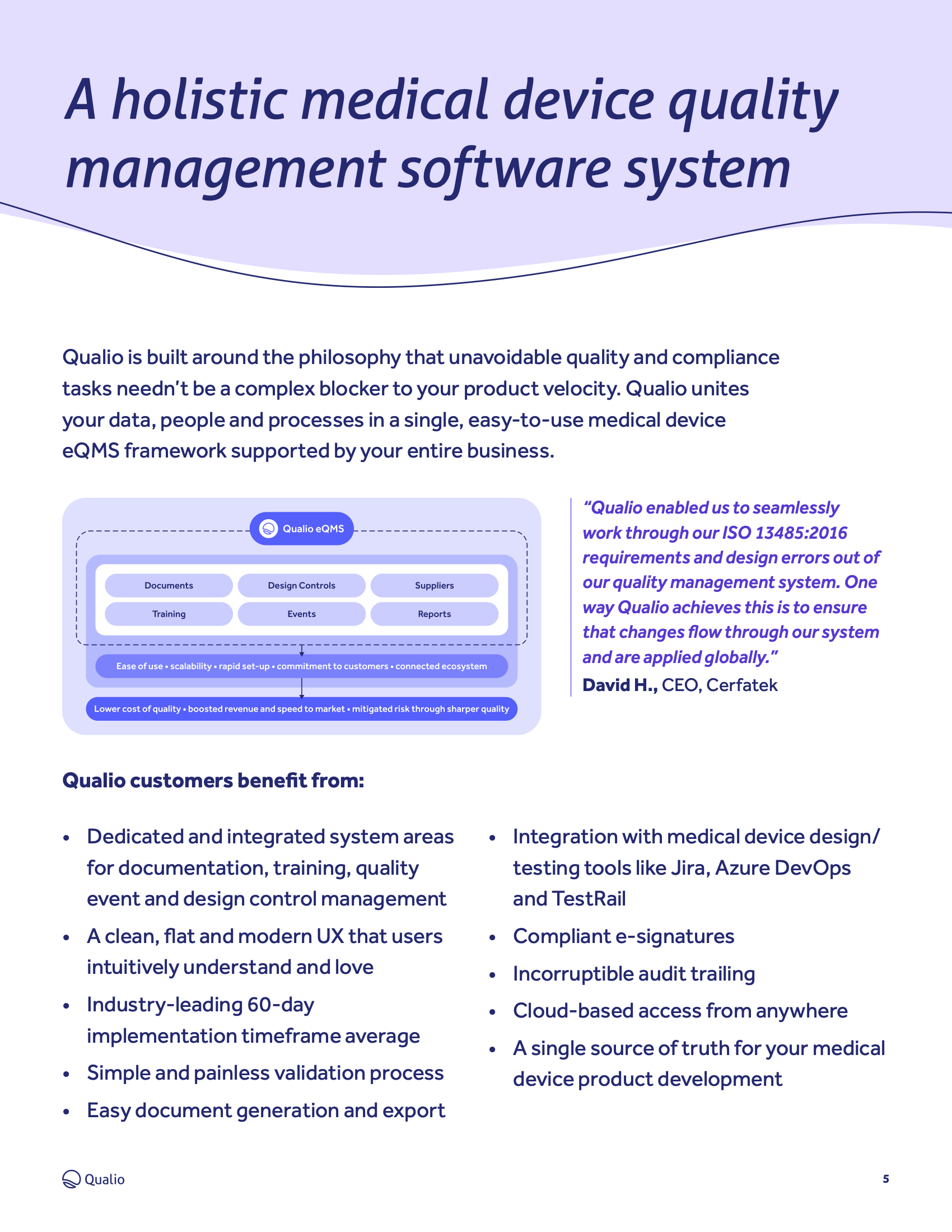
Medical device quality management software datasheet