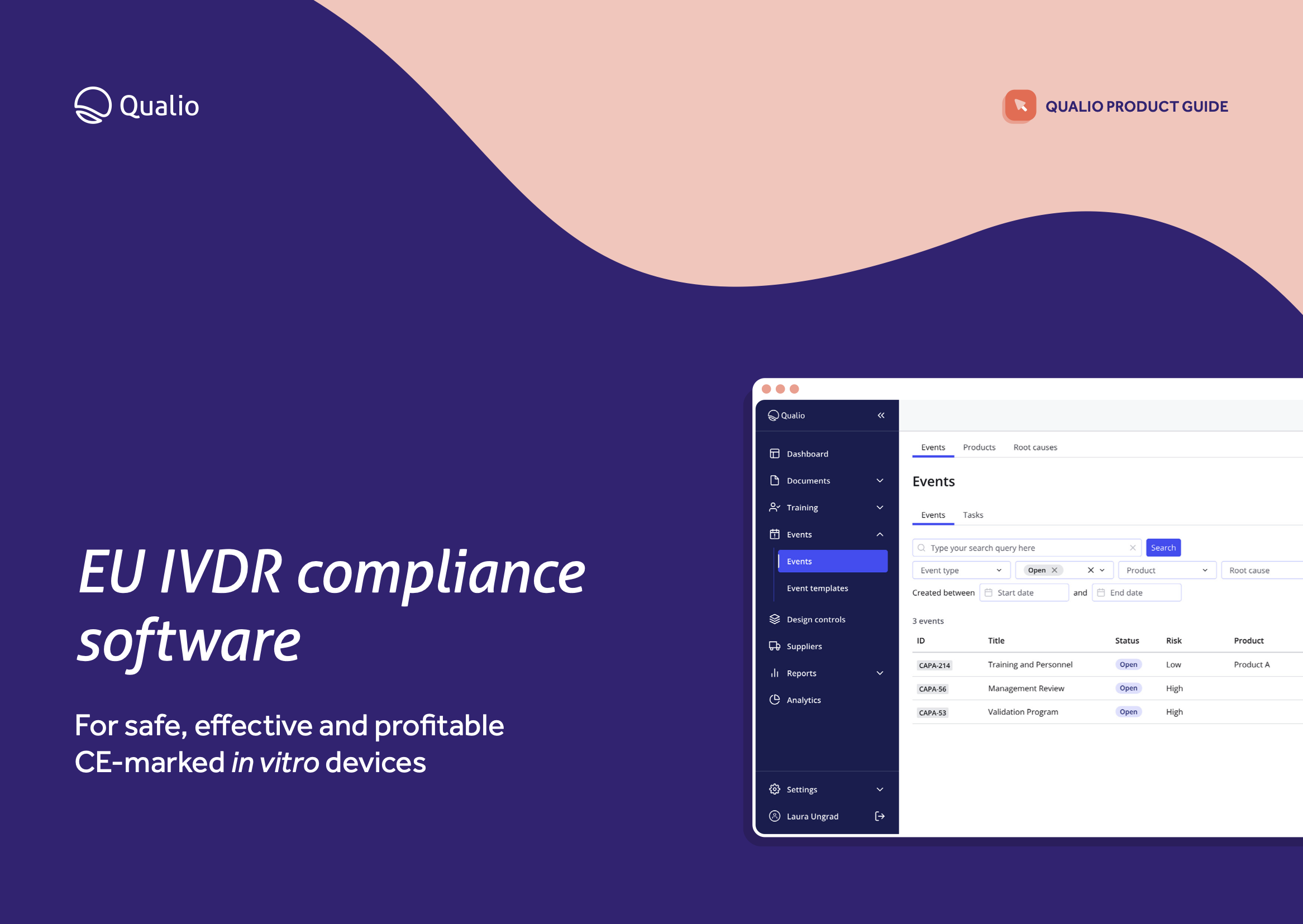
EU IVDR software datasheet
Learn how our software gives you the tools, traceability and control you need to unlock the EU market for your in vitro diagnostic device.
Download our EU IVDR datasheet to learn how Qualio:
-
Helps your business meet the requirements of the EU IVDR in a single digital quality system
-
Accelerates your route to the EU market by connecting your teams to a holistic eQMS
-
Makes your IVD business constantly compliant and audit-ready
Complete the form to the right to get started!
What you'll get:
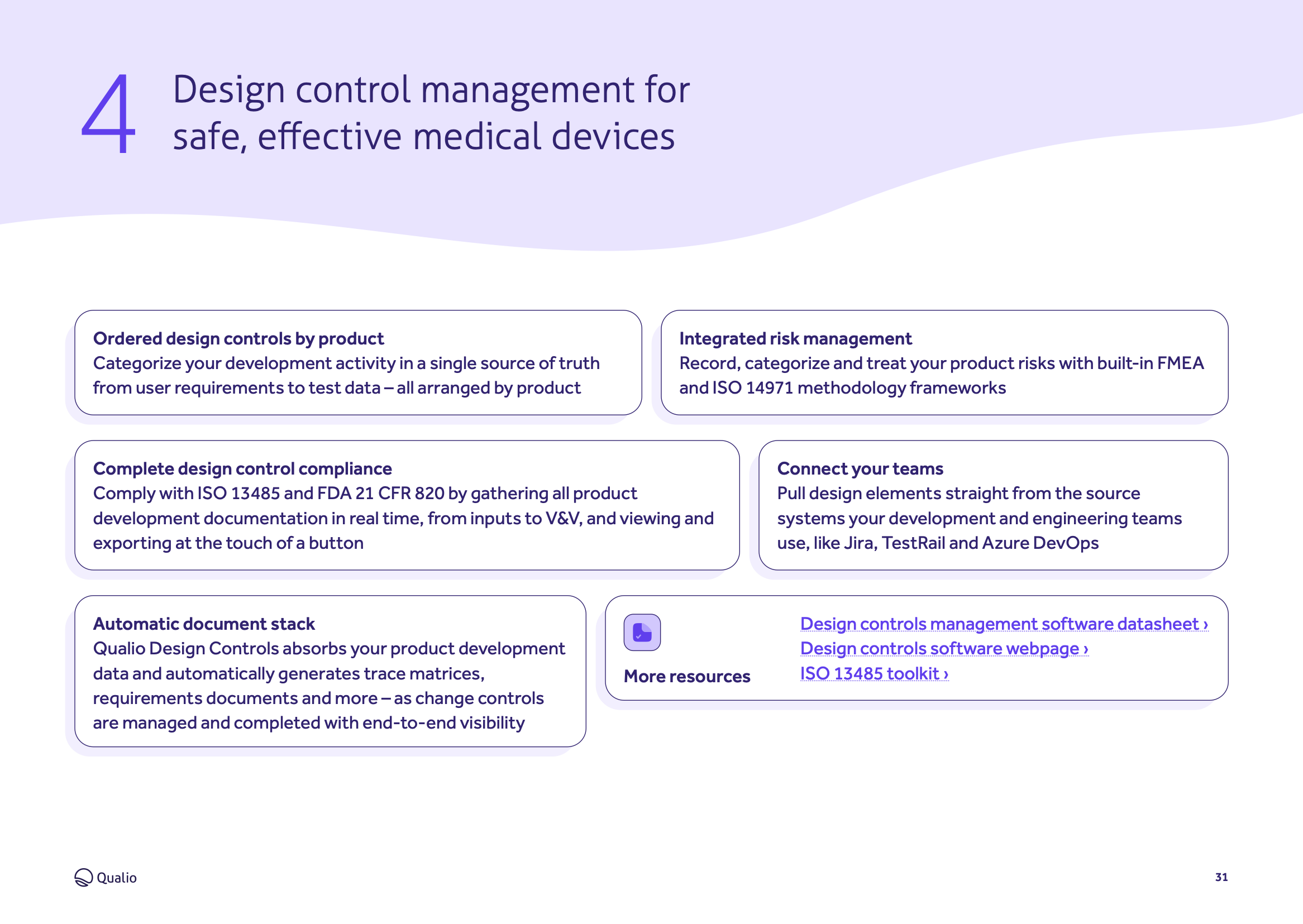
Feature breakdown
Explore the core features and functionality of Qualio, and how each area of the system contributes to a holistic eQMS for in vitro diagnostic medical device organizations
Complete IVDR compliance
Dive into how Qualio's features map to each requirement of the EU IVDR and empower your business with complete compliance
IVDR eQMS blueprint
See how real Qualio customers unite document, training, event, design control and supplier management to get to market and scale successfully