Medical device quality management software datasheet
Learn why hundreds of medical device companies use Qualio for industry-leading quality.
Download our medical device quality management datasheet to learn how Qualio:
- Arms your business with a complete, compliant eQMS for medical device quality management
- Supports and simplifies compliance with the demands of FDA 21 CFR 820, ISO 13485, the EU MDR, and more
- Digitizes and automates all areas of modern medical device quality management, from design controls to document management
Complete the form to the right to get started!
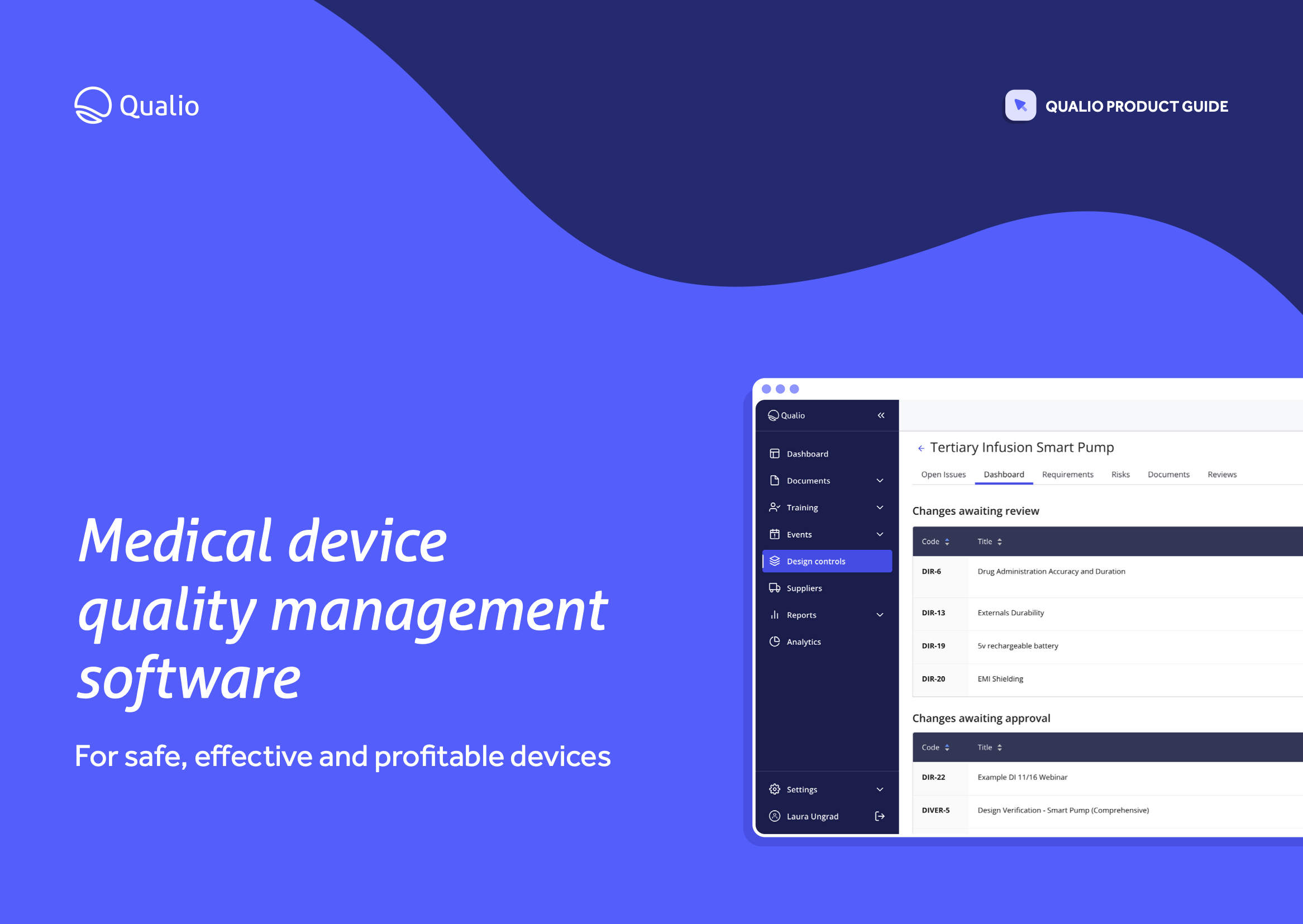
What you'll get
Feature breakdown
Explore the core features and functionality of Qualio, and how each area of the system contributes to a holistic medical device eQMS
Total compliance
Learn how Qualio meets the demands of ISO 13485, the medical device quality management standard, and all other national standards
Set-up, services and more
Hear from real Qualio medical device customers, learn how we onboard and implement our software, and access more helpful resources