Using Qualio for building a medical device technical file
Technical files are complex, time-consuming compliance hurdles for medical device companies on their way to market.
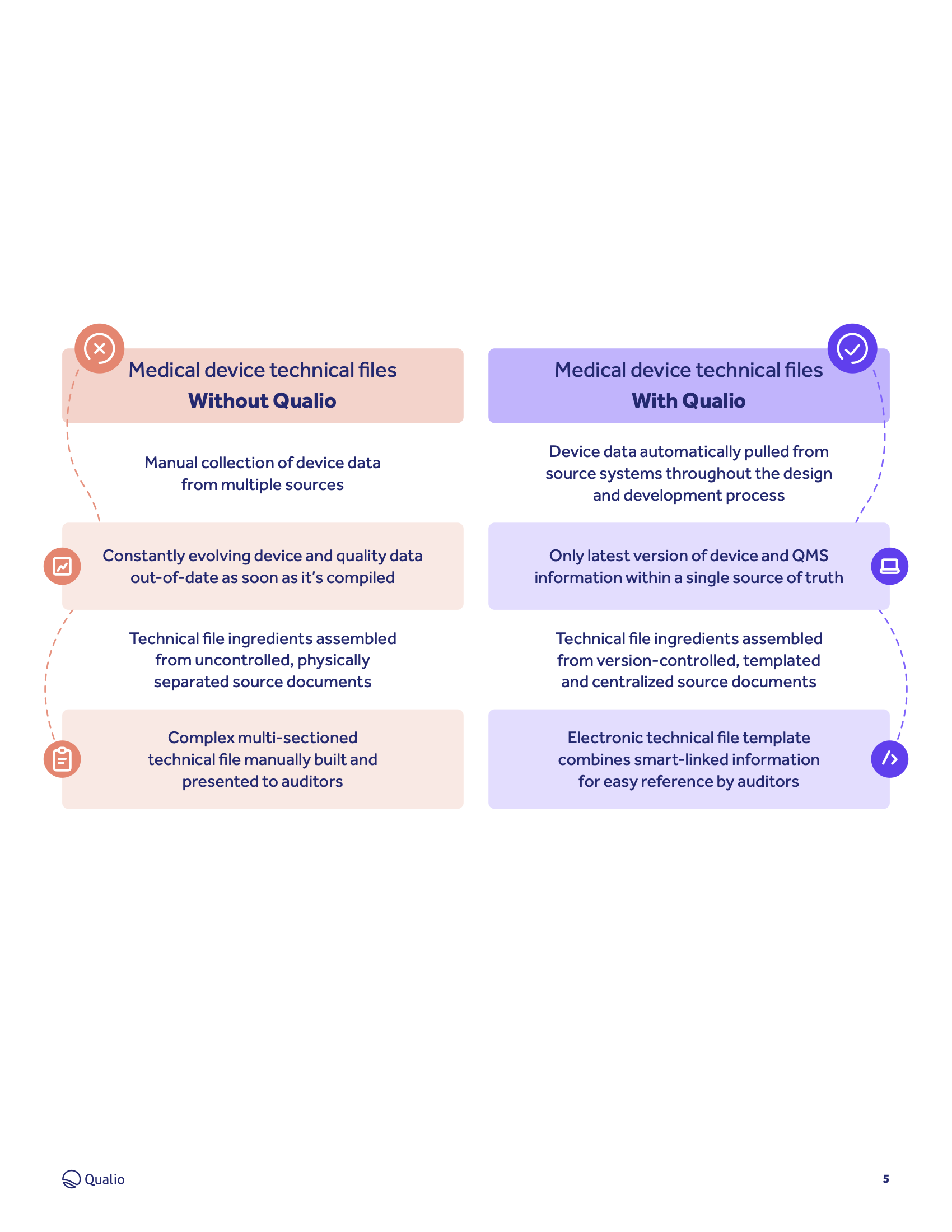
Learn how Qualio makes it quick and easy to build, collate and present your technical file data.
Download our medical device technical file guide to:
- See how real medical device companies use Qualio to assemble technical files for compliance with FDA 21 CFR 820, ISO 13485, the EU MDR and more
- Learn how Qualio makes it quick and simple to build design history files, device master records and medical device files with all their respective ingredients
- Build and present a logical, structured and fully paperless medical device technical file that unlocks market access for your device
What you'll get:
Blueprint for smarter technical file builds
Learn how Qualio medical device customers replace cluttered, uncontrolled paper and spreadsheets with templated, cloud-based and always-compliant medical device technical documents
Product deep dive
Understand how Qualio's documents and design controls functionality combine to accelerate, automate and simplify your technical file creation
Simplified medical device compliance
You'll need a robust and auditable technical file for ISO 13485, FDA and EU compliance. But that doesn't need to take months of work and manual upkeep. Explore how to use Qualio's tags, templates, dashboards, document editor and more to make your life easier and get to market without stress