SOFTWARE VALIDATION
Rapid, compliant validation
without the headache
A modern, best practice assurance approach that gets you
up and running faster than any other eQMS

Embracing computerized system assurance
Challenge
eQMS vendors continue to push traditional computerized system validation (CSV) approaches onto their customers.
Fear of regulatory punishment and reliance on outdated documents slow time to value and bog software customers down in stressful, time-consuming, and often unnecessary validation of every single software feature.
Solution
Qualio's modern computerized system assurance (CSA) approach does the bulk of the heavy lifting for you, freeing up your time for targeted and 'least burdensome' assurance completion.
Modern, appropriate document templates, a validation pack and a sensible, efficient shared roadmap minimize time and effort.
Less time on admin, more time getting value
- Go live with Qualio 2 weeks faster than traditional methods
- Receive assurance templates and automated document packs to accelerate or eliminate tasks
- Enjoy more time using Qualio, sharpening your quality and focusing on your higher-risk software systems
CSA, not CSV
- Modern validation approach based on the latest FDA and ISPE GAMP guidelines
- Designed with minimal customer effort in mind
- Appropriate, 'least burdensome' activity only!
Ditch old, time-consuming tasks
- Make IQs, OQs, PQs and onerous system testing a thing of the past
- Enjoy a fast, efficient assurance approach with no wasted activity or outdated documents
- Start using Qualio 50% faster than
any other eQMS
Be confident in your compliance
- Fully aligns with industry expectations of modern, optimized software validation
- Reviewed and approved by the editor of GAMP 5
- Automated re-assurance packs keep you compliant with every new system release
Validation resources
3 top benefits of our validation approach
Less time validating
No more testing timesinks.
CSA tasks are weaved into your Qualio onboarding as you build and configure your system, getting you off the starting line quicker.
Sensible, appropriate work
Qualio is a low-risk, non-product system.
Our efficient, risk-based approach takes this into account, leaving you free to focus on your higher-risk systems.
High-touch support
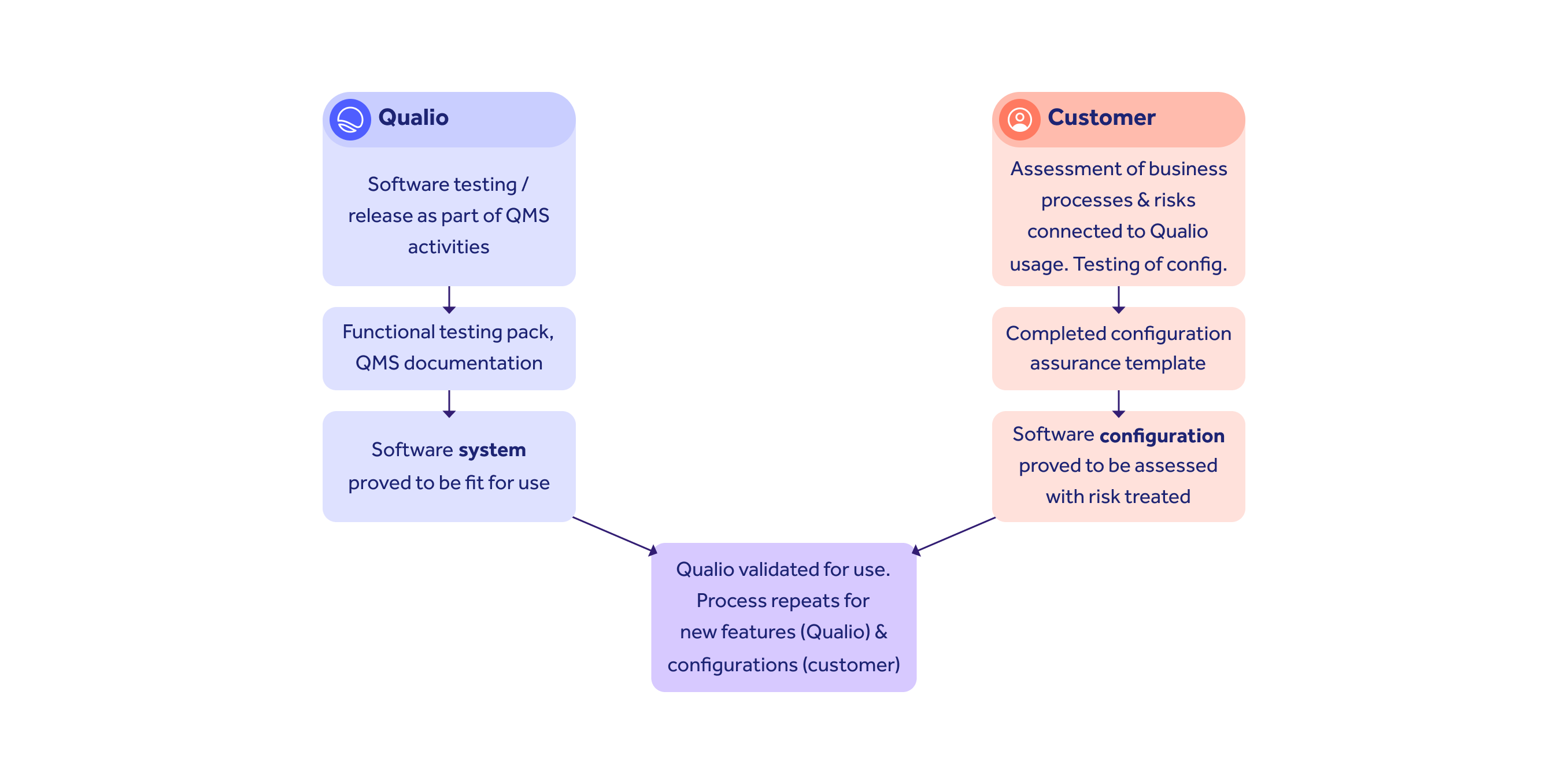
CSA divides responsibilities between system (vendor) and config (customer) assurance.
Qualio provides templates and expert support to help you complete your tasks without fuss.



