2025 guide to pharmaceutical software
Pharmaceutical software is no longer an optional tool for forward-looking drug manufacturers.
As industry bodies like the FDA and ICH increasingly expect optimized, digitized and accelerated operations, pharma software has become part and parcel of how modern pharmaceutical companies should go about their business.
From automating quality management tasks to sharpening manufacturing and resource allocation, pharmaceutical software has revolutionized the entire drug lifecycle from R&D to distribution and pharmacovigilance - at least, for those businesses shrewd enough to harness it.
If your business plans to stick around and stay competitive in the future, a timely and smart pharmaceutical software investment should be high on your to-do list.
We've built a list of the top pharmaceutical software system types your company should explore and onboard in 2025.
Table of Contents
- What is pharmaceutical software?
- Benefits of pharmaceutical software
- What software tools are used in the pharmaceutical industry?
- The future of pharmaceutical software in 2025 and beyond
What is pharmaceutical software?
Software-as-a-service (SaaS) platforms have already unleashed transformative changes in industries such as shipping, payroll, finance and logistics.
Life science, and particularly pharmaceutical businesses, have been slow to catch up and keep pace.
Qualio's 2023 life science quality trends report found 49% of life science companies still relying on paper and spreadsheets for their vital quality and compliance operations.
But things are changing. The development of software for pharma companies has emerged with the goal of transforming complicated, disconnected and manual industry processes into digitized, connected, collaborative and automated ways of doing things.
Pharma software, in short, is any package of software that digitizes and automates traditional analog tasks connected to a pharmaceutical operation.
And because the pharma world operates very differently to, say, food or hospitality, pharmaceutical software refers to the specialized software solutions designed to address the unique needs and challenges of the pharmaceutical industry.
Benefits of pharmaceutical software
Pharma software encompasses a wide range of applications and use cases, from supply chain and laboratory management to supporting quality assurance and standardizing manufacturing processes.
Pharmaceutical software doesn't only boost your operational efficiency by streamlining operations and reducing manual tasks. It can also improve data security and integrity, making ALCOA+ adherence easier by safeguarding documents and data.
By centralizing information from scattered paper and spreadsheet records into a dedicated digital platforms, users enjoy access to more accurate, controlled and real-time data for informed decision-making.
And robust cGMP becomes easier too, as pharmaceutical software and connected tools boost both automation and consistency:
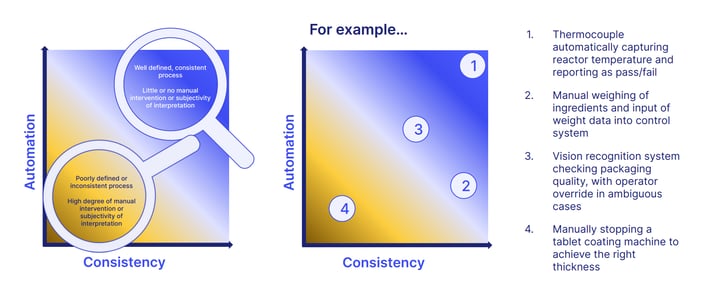
Because of these benefits, the industry increasingly expects pharma companies to embrace pharma software to stamp out the repeating compliance issues plaguing the industry.
The FDA's nascent quality management maturity program had its 5 core tenets mapped out in an August 2023 whitepaper.
Number 4? Technical excellence.
For the FDA, this includes...
"...implementing innovative manufacturing processes or novel solutions... using advanced technologies that are fit for purpose... [and] determining a proposed solution (e.g., technological upgrade of equipment or software)..."
Pharmaceutical software = a mature, robust and continuously improving pharmaceutical operation.
What software tools are used in the pharmaceutical industry?
With all that in mind, let's explore the main types of pharmaceutical software any serious pharma business needs in 2025.
Pharmaceutical ERP software
Pharmaceutical ERP software refers to any digital tool that manages enterprise resource planning (ERP) within a pharmaceutical business context.
Pharma ERP software provides an integrated and connected view of your key business processes and databases, such as your manufacturing capacity, finished drug lots and batches, raw materials, cash, purchase orders, payrolls and so on.
Since control and traceability of components, drug product containers and closures is a key cGMP requirement mapped out in FDA 21 CFR Part 211, pharmaceutical ERP software is a great way to more easily keep track of your inventory, stock, active pharmaceutical ingredients (APIs) and packaging as it flows into and out of your business.
The best software for pharma ERP integrates data and processes from different departments such as finance, procurement, HR, manufacturing, supply chain, sales and customer service. This integration helps ensure that resource data is consistent and accessible across your organization.
Pharmaceutical ERP software examples
Pharmaceutical manufacturing software
Pharmaceutical manufacturing software is a broad category of software tools which support the manufacture of pharmaceutical product.
Process analytical technology, or PAT, is a crucial subset of pharma manufacturing software which makes both cGMP and ICH Q8 quality by design easier to embed.
Since QbD works by the setting and measuring of critical quality and performance attributes connected to your product, PAT pharma manufacturing software helps you design, analyze and control your pharma manufacturing by measuring, tracking, trending and reporting on those attributes.
In turn, you get the insight and notification you need to take action if your attributes should stray from their acceptable limits ('design space') and threaten the integrity and efficacy of your drug product.
PAT pharma manufacturing software tools include real-time monitoring and analysis tools like imaging systems, spectroscopic platforms and chromatography.
Another pharmaceutical manufacturing software subset is the manufacturing execution system, or MES.
MES platforms form a kind of bridge between your pharmaceutical ERP software system, which operates at a high-level strategic resource focus, and something like a supervisory control and data acquisition (SCADA) platform, which supervises on a more tactical level how your machines and processes work and mesh in real time.
The MES, in short, tracks and monitors the flow of APIs through your manufacturing system into their finished dosage forms (FDFs). For this reason, it's a key example of software for pharma companies looking to optimize the quality and integrity of their outputted product.
By analyzing and monitoring your manufacturing execution, MES pharmaceutical manufacturing software allows resource scheduling, equipment and product quality analysis, production and downtime measurements, and drug lifecycle tracking. Since MES platforms typically generate electronic manufacturing histories, they're particularly valuable for outputting electronic batch records - a crucial pharmaceutical cGMP requirement.
They also simplify the drug amount reporting obligations mapped out for US pharma companies in the 2020 CARES Act.
A key consideration when automating your GMP manufacturing processes with pharmaceutical manufacturing software is GAMP: good automated manufacturing practice.
Because pharma manufacturing software is intimately connected to the safety of your product, you can't just go ahead and put any software system into your manufacturing line straight away. A bug or untested feature could wreak havoc.
You'll need to validate and prove the suitability and integrity of your pharmaceutical manufacturing software system first - preferably with the agile, risk-based best practice mapped out in the Second Edition of GAMP 5 and the FDA's latest computer system assurance guidelines.
Download our complete guide to computerized system compliance in 2023
Pharmaceutical manufacturing software examples
Pharmaceutical CRM software
CRM, or customer relationship management, is a vital discipline for businesses dealing with customers in any industry.
Pharmaceutical CRM software is therefore an important investment for any pharma business that's marketing a product and/or service, and interacting with a customer base at scale. (Contract research and manufacturing organizations included.)
Software for pharma CRM isn't particularly niche or unique in the way that pharmaceutical ERP software or pharmaceutical manufacturing software is. Although pharma companies should demonstrate good, documented control of their third-party relationships, there aren't many pharma-specific regulatory obligations and standards for how to do this. Customer relationships are, broadly, customer relationships.
Although some CRM tools (like one of our own customers, StayinFront) are geared more towards pharmaceutical customers, industry-agnostic CRM software could therefore also be okay for pharma companies to use, provided it has key functionality like:
- Customer database and contact information
- Sales/marketing campaign automation
- Sales process and interaction tracking and tracing
Pharmaceutical CRM software examples
Pharma supply chain software
Pharma supply chain software has only grown in significance in the post-COVID age. The modern pharmaceutical supply chain is longer, riskier, more complex and more fragmented than it's ever been. Software for pharma supply chain management, then, is a crucial tool to consider.
The pharmaceutical supply chain encompasses the entire journey of your drug products from manufacturers to end users, including via pharmacies and hospitals to patients. Stringent quality control, temperature-sensitive requirements and good distribution practice (GDP) compliance should all be considered and continuously applied throughout.
There's considerable crossover here with some of the other software for pharma companies that we've touched on above.
Pharmaceutical ERP software, for instance, typically contains the inventory management, order processing and demand forecasting functionality needed as part of pharma supply chain management.
A pharmaceutical CRM software system, meanwhile, would allow you to build and interact with a database of supply chain customers like CMOs, distributors and so on.
But other key pharma supply chain functionality should be sourced from a dedicated system, such as:
- Supplier onboarding and assessment
- Traceability and serialization of distributed product
- Any temperature and conditional monitoring systems needed for your product as it's moved
You may require multiple, interacting pharmaceutical supply chain software and pharmaceutical distribution software systems to fulfil your operational and regulatory demands, and you should therefore treat this category as a broader umbrella of software options rather than a single system to tick off.
Get the guide: how to manage your life science suppliers like a pro
Pharma supply chain software examples
Pharmaceutical compliance management software
Pharmaceutical compliance is hard.
Alongside cGMP basic requirements, your organization is exposed to a plethora of other contextual GxP expectations, from GDP as your product is distributed to GCP if you're a clinical operation. That's not to mention GDocP and ALCOA+ document integrity expectations, GLP if you run a laboratory (more on that below), and even GVP for your post-market pharmacovigilance activities.
Pharmaceutical compliance management software, also known as pharmaceutical quality management software, offers a range of holistic functionality to help make both regulatory compliance and optimized product quality natural and automatic.
Here's an example of the operational areas managed within Qualio, a leading pharmaceutical compliance management software system:
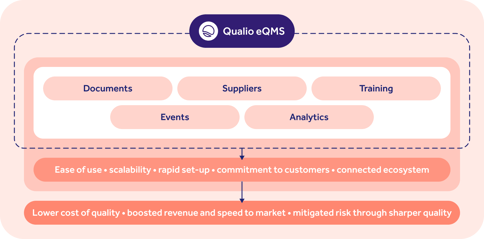
Typical pharmaceutical compliance management software features include document, training and event management functionality, among others.
Pharmaceutical compliance management software assists in the creation, organization and maintenance of essential compliance and quality documentation, such as standard operating procedures (SOPs), work instructions, quality management records and regulatory submissions, all underpinned by automatic audit trails and FDA- and EU-compliant e-signatures.
The best software for pharma compliance also tracks and manages employee training and competency records, ensuring that staff are adequately trained and qualified for their roles while allowing ongoing training to be cascaded and completed without time-consuming wet signature processes.
Pharmaceutical compliance management software should also include configurable event management functionality, allowing digital workflows to be built and executed to close out events like out-of-specification investigations, deviations, complaints, CAPAs and more.
And here, too, there's crossover with pharma supply chain software systems. Strong pharmaceutical compliance management software systems typically offer supplier management functionality too, including onboarding assessments, risk-based scoring, documentation libraries and audit cadence-setting. A shrewd compliance software investment can therefore tick off multiple pharma software requirements in one go.
Users of pharmaceutical compliance management software like Qualio report accelerated time to market, dramatically reduced admin time, more time for proactive continuous improvement, fewer defects and faults and, as a result, stronger and more embedded regulatory compliance.
Pharmaceutical compliance management software examples
Laboratory information management system (LIMS) software
Our final pharmaceutical software recommendation is the laboratory information management system, or LIMS.
Running a laboratory? You'll need a LIMS software system on your pharma software shopping list.
LIMS platforms originally emerged as sample management systems for tracking and tracing samples across a lab operation. But they've since matured and expanded to encompass other functionality, such as:
- Instrument calibration and maintenance
- Import/management of assay results
- Electronic data exchange and 'smart' connection of lab systems
- Reagent and lot tracking
- Digital workflows for actions like sample processing and data input
And more.
LIMS platforms ease the compliance burden of Good Laboratory Practice and help make repeatable, standardized GLP part of how you do business.
As the fully digital laboratory becomes less of a dream and more of a reality, investing in a LIMS now is a great way to accelerate and strengthen your laboratory operation.
Laboratory information management system (LIMS) software examples
The future of pharmaceutical software in 2025 and beyond
Pharmaceutical industry software continues to evolve into new and exciting iterations. The world of software for pharma companies in 2025 promises a richer, more connected and fully digital pharmaceutical approach.
Cloud-based solutions
Cloud-based pharmaceutical software offers a nimbler, simpler way for pharma companies to digitize.
Older on-premise software systems demanded high levels of investment in physical software support infrastructure like servers, data centers and networks, with internal IT expertise to run it all.
Critical upkeep tasks like upgrades, hosting, security and patches fell to the customer.
And access was restricted to on-site logins: a major headache for the increasingly remote and distributed post-COVID pharmaceutical world.
Cloud-based pharma software changes all that, allowing customers to jettison physical on-premise costs, offload upkeep tasks to their vendor and access their software systems from anywhere in the world with an Internet connection - making collaborative work and research that much easier.
The role of AI and machine learning
AI seems to be everywhere, doesn't it? Pharmaceutical software is no different.
Drug discovery is a long, ludicrously expensive and punishing process - only 10% of drugs make it through to human clinical trials.
Machine learning and AI holds the key to accelerated, safer drug discovery processes, with tools like AI-powered 'decision engines' allowing prospective drugs to be modelled and tested virtually and 'in silico' at scale before making their way into a human body.
Listen to our podcast episode: how AI can accelerate drug discovery
Other applications offer exciting upgrades to pharmaceutical users.
Predictive maintenance pharma software tools could boost equipment effectiveness, automating calibration tasks and slicing downtime.
Quality and regulatory content could be created at lightning speed by an AI writer, integrating product data in real time to give users an audit-ready document stack in seconds.
And AI modelling could help predict where drug shortages, demand spikes and disease outbreaks occur, allowing smarter and more targeted delivery of product to patients.
Other trends to watch
The primary pharmaceutical software trend to watch in 2025 is its continued expansion.
To remain competitive, pharma businesses simply cannot afford to stick with legacy, analog and paper-based ways of working.
Pharmaceutical software systems, from pharmaceutical ERP software to pharma supply chain software, promise your business faster, easier progress to your operational goals, embedding lasting compliance and giving you new time and visibility to optimize your business.
At the same time, be on the lookout for those pharma software systems that tick off multiple pharmaceutical software requirements in one go.
The category list we've assembled for this blog post is becoming less and less mutually exclusive, as pharma software vendors broaden their functionality offerings to become more of a 'one-stop shop' for their customers.
A manufacturing execution system could contain ERP functionality, too. Pharmaceutical compliance management software like Qualio also ticks off pharma supply chain management functionality. And there's no reason your LIMS, SCADA and PAT platforms can't connect and integrate.
The pharmaceutical companies that thrive in 2025 will be those with a proactive, energetic approach to pharmaceutical software investment.
