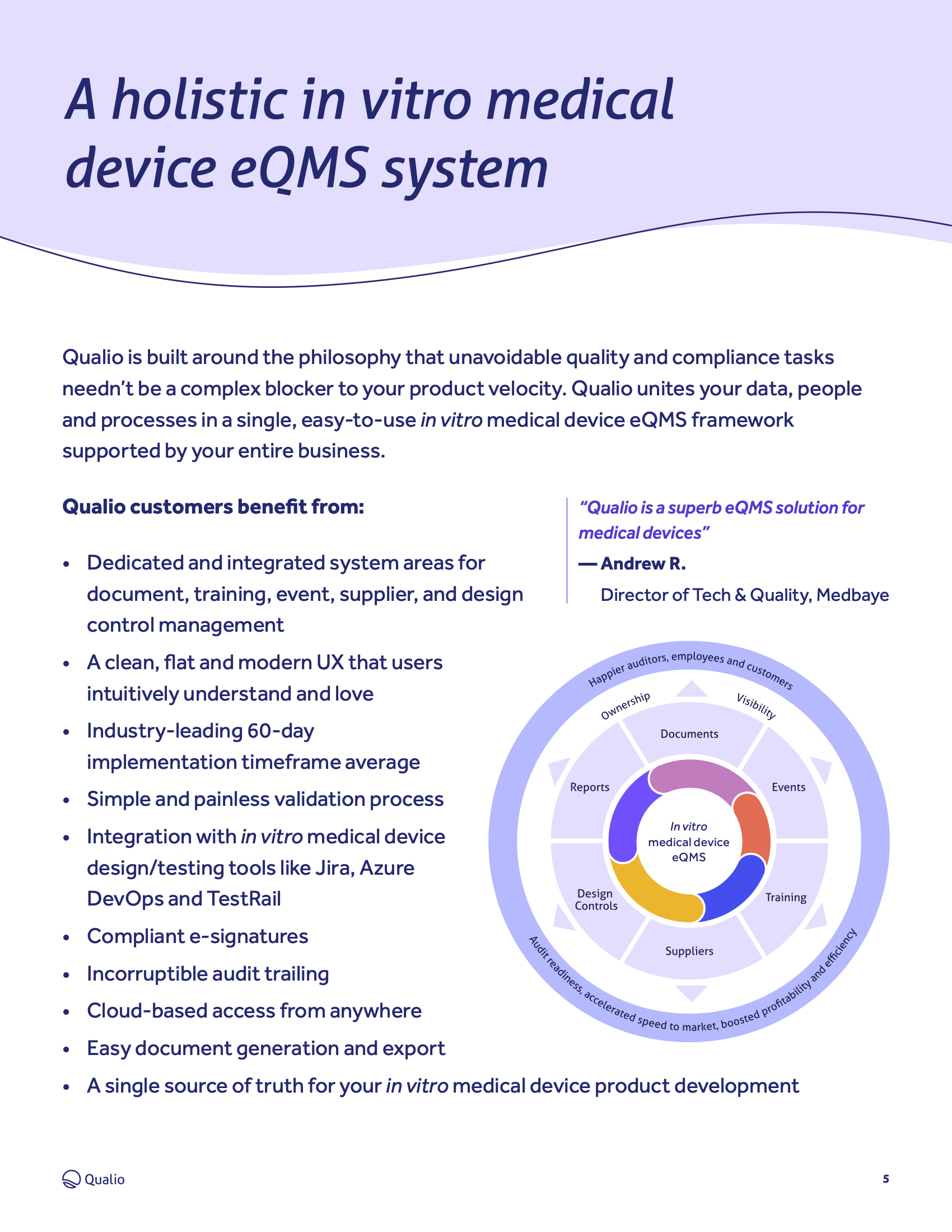
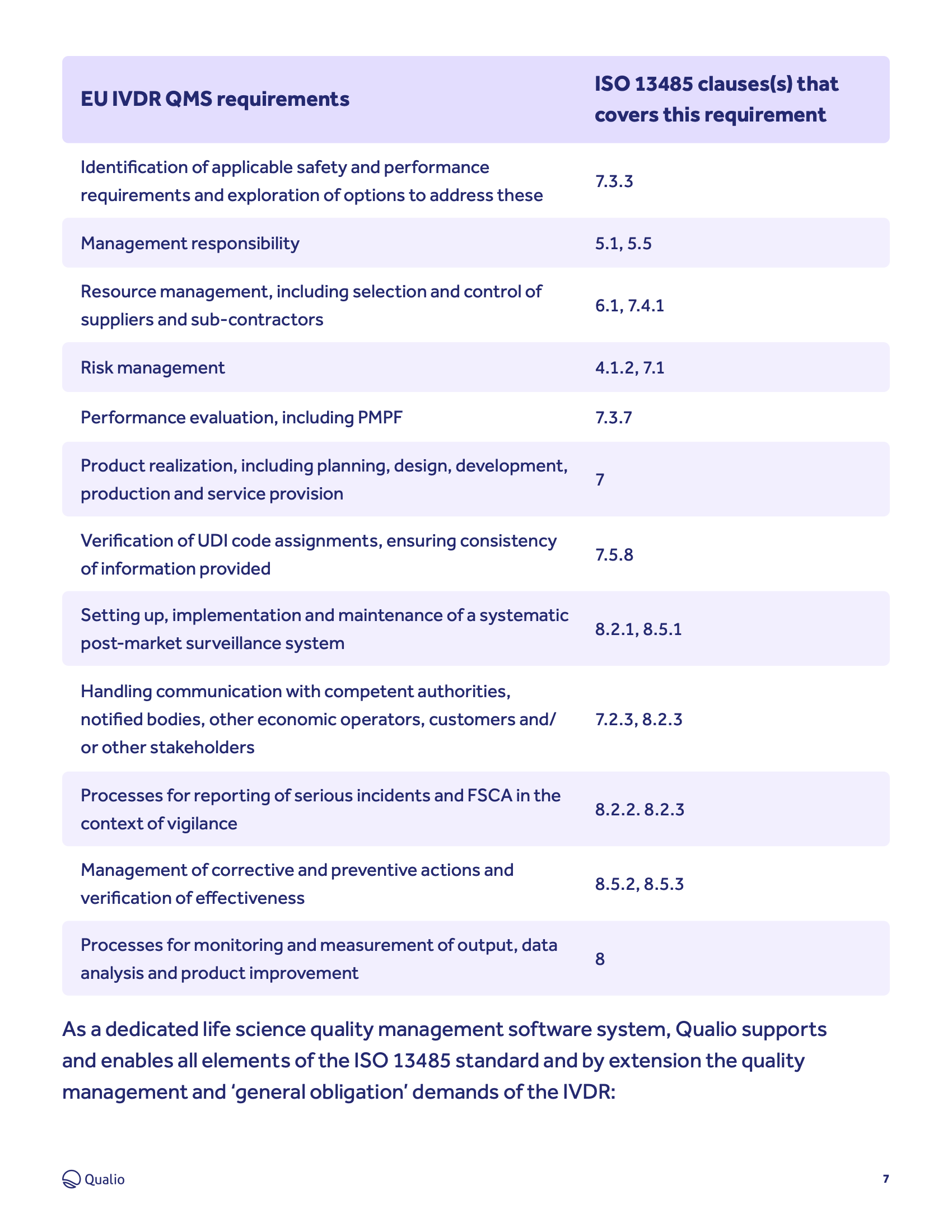
IVDR compliance: tips and best practice
Watch this webinar to:
- Learn about the key requirements of the EU's In Vitro Diagnostic Regulation (IVDR) as it comes into effect
- Understand the key actions your business needs to take for full compliance
- Access compliance tips and tricks for a smoother, stress-free CE marking journey
What you'll learn
- IVDR expertise
Hear Qualio's Kelly Stanton and Sumatha Kondabolu share tips and suggestions based on their extensive medical device quality management experience - Requirements breakdown
Take a deep dive into exactly what the IVDR demands and get to grips with how to unlock the European market - Actionable compliance pathway
Learn what your business needs to do to get your product CE marked and approved under the IVDR - and how you should start your compliance journey