Good Manufacturing Practices (GMP) explained
If you make a sensitive, regulated product, Good Manufacturing Practices, or GMP, are critical to your operation.
GMP is a deep and complex topic, so we assembled this complete guide to help you orientate.
Let's dive into GMP and what it means for your business.
Introduction to Good Manufacturing Practices (GMP)
In industries like pharmaceuticals, cosmetics and medical devices, ensuring product quality and safety is of utmost importance.
Manufacturers must adhere to strict standards that govern every aspect of production to prevent risks such as contamination, incorrect labeling or suboptimal quality.
One such set of standards is that of Good Manufacturing Practice (GMP). These are a globally recognized set of regulations that aim to ensure that products are consistently produced and controlled according to quality standards.
GMP meaning
GMP, then, refers to the practices required to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of products such as cosmetics, pharmaceuticals and medical devices.
The guidelines ensure that products are safe for use and that they have the attributes they claim. GMP covers all aspects of production from the raw materials, premises, and equipment to the training and hygiene of staff.
What does GMP stand for?
GMP stands for Good Manufacturing Practice - or sometimes Practices.
These practices are designed to ensure that products are consistently produced and controlled to meet quality standards. The focus is on the processes and environment in which products are made, and not just on the final quality assurance of the product itself. It aims to minimize the risks involved in any production process that cannot be eliminated through final product testing.
You'll sometimes see a 'c' before 'GMP'. 'cGMP', or 'current' good manufacturing practice, is a term particularly applied in the United States to reflect the ever-evolving and shifting nature of manufacturing best practice and regulatory expectations.
It's important to note, too, that GMP sits within the broader umbrella of GxP.
GxP is the overarching category of 'good practice' which governs the quality expectations of modern regulated industries.
The 'x' is interchangeable - with 'M' obviously standing for 'manufacturing', but with other GxP subsets like GCP ('good clinical practice') or GLP ('good laboratory practice') also defined and mandated for certain industries.
It's often the case that multiple GxP groups will be applicable for your organization - a pharmaceutical manufacturer, for instance, would be expected to demonstrate GMP adherence as well as other benchmarks like GDocP ('good documentation practice') or GVP ('good pharmacovigilance practice').
What are the 5 Ps of GMP?
The so-called '5 Ps' of GMP are a concept borrowed from the broader GxP category.
These are people, products, procedures, processes and premises.

The 5 Ps aren't an official term, and you won't see any regulations structured in this way.
But they're a useful way to think about your GMP planning, and the key operational areas you'll need to focus on for full GMP compliance.
People, for instance, references the importance of training and competence among your workforce.
Products are really the core focus of GMP: is your product safe, effective and what it's supposed to be?
Procedures relate to the importance of properly documenting and structuring your operation to ensure good manufacturing practice is traceable and embedded everywhere.
Processes are how things get done in your business, and baking quality and compliance into how your processes are designed is a cornerstone of GMP adherence.
Lastly, your premises are critical as the physical space where all this stuff takes place. Cleanliness, hygiene, order and access are key things to consider in this category.
We'll explore GMP requirements in more detail below.
Importance of GMP
The importance of GMP cannot be overstated. Poor manufacturing practices can result in contamination, incorrect dosages, faulty products, adulteration or mislabeling. Such errors can lead to serious consequences, including harm to consumers and legal liabilities for you, the manufacturer.
GMP sets the foundation for quality assurance and consumer protection by emphasizing prevention rather than detection of errors through quality by design.
Furthermore, GMP plays a crucial role in standardizing international quality expectations. Many countries require products to be manufactured under GMP-compliant conditions for them to be imported. GMP compliance helps ensure that products are consistently produced and controlled according to international standards, promoting global business relationships and consumer trust.
Standards and guidelines
It's important to note that GMP isn't a single regulatory benchmark.
GMP standards and guidelines aren't universally fixed, but are subject to interpretation by various regulatory agencies around the world. These agencies, such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO), all have their own specific GMP guidelines.
The FDA, for instance, mandates compliance with FDA 21 CFR Parts 210 and 211 to ensure GMP is met within the United States.
The EU's EudraLex Volume 4 maps out the GMP expectations of the EMA.
And the WHO's TRS 986 Annex 2 is often applied by pharma companies across Asia and Africa.
However, the core principles of GMP remain consistent across these different jurisdictions.
These include ensuring hygiene, preventing cross-contamination, documenting processes, and implementing quality control measures at every stage of production.
What are GMP requirements?
GMP encompasses a wide range of requirements designed to ensure product safety and quality. These requirements focus on everything from personnel training to facility cleanliness and raw material handling.
Some GMP guidelines, like ICH Q7, even focus on specific products, such as active pharmaceutical ingredients (APIs).
Here’s a detailed breakdown of some core GMP requirements:
Quality management
Quality management forms the backbone of GMP. It involves establishing a quality management system that ensures products are designed and manufactured to meet the required specifications. A robust quality management system (QMS) includes policies and procedures that govern every aspect of production, from supplier qualification to final product release.
The QMS should also include regular internal audits to evaluate whether the GMP standards are being followed. If non-compliance issues are found, corrective and preventive actions (CAPAs) must be put in place to address and prevent them from recurring.
Above all, the QMS needs to integrate and link all GMP activity with the wider business, ensuring it's properly embedded across the product lifecycle and connected with the good laboratory or good clinical practice that precedes it:

Consistency and control
Consistency in manufacturing is key to ensuring that your products meet the required GMP standards. GMP requires strict control over every phase of production, including the sourcing of raw materials, the operation of machinery, and the handling of the finished product. This means that every batch of product must be made under the same conditions using the same procedures to guarantee uniformity and quality.
Control also applies to environmental factors like temperature, humidity, and cleanliness, which can all affect product quality and, in the pharma world, the 'critical quality attributes' of your product.
These environmental factors must be carefully monitored and documented to ensure GMP is in place - and pharma companies are increasingly turning to digital tools to help them embed this activity at scale.
GMP training
Remember the first of the '5 Ps' above?
People - your employees and colleagues - play a vital role in ensuring GMP compliance. GMP training is mandatory for all personnel involved in the production process, including operators, quality control staff, and even maintenance workers. This training must be continuous and updated regularly to reflect new regulations or procedural changes.
Training programs typically focus on hygiene practices, equipment handling, process documentation, and emergency procedures. Proper training ensures that your staff understand their role in maintaining GMP standards, and minimizes the risk of human error.
Equipment and maintenance
GMP guidelines require that all equipment used in the production process is designed, maintained and frequently calibrated to operate consistently and reliably. Equipment should be regularly inspected for wear and tear, and maintenance should be scheduled to prevent breakdowns during production.
Faulty equipment can lead to product contamination or inconsistency, which could compromise the entire production run.
A preventive maintenance plan should therefore be implemented, and all maintenance activities should be logged and reviewed periodically for inspection by quality teams or by external auditors.
Raw materials
GMP guidelines place particular emphasis on the quality of raw materials used in production. After all, you can't get a safe, compliant product with non-compliant ingredients.
All raw materials must be verified for quality before they enter the manufacturing process. Suppliers should be vetted and qualified, and incoming raw materials should be quarantined until their quality has been verified.
Additionally, raw materials must be stored under appropriate conditions to prevent contamination or degradation. Proper labeling and documentation of raw materials are essential to trace any issues back to their source in the event of a product recall or quality investigation.
Documentation
GMP compliance is heavily reliant on accurate and detailed documentation. Every step of the production process, from raw material sourcing to final product distribution, must be documented. This documentation provides traceability and ensures that all manufacturing activities are performed according to pre-approved procedures.
Document control systems should be in place to manage the creation, review, approval, and storage of records. Documents such as batch records, standard operating procedures (SOPs) and quality control reports must be readily accessible for review during audits.
Typical GMP documents include:
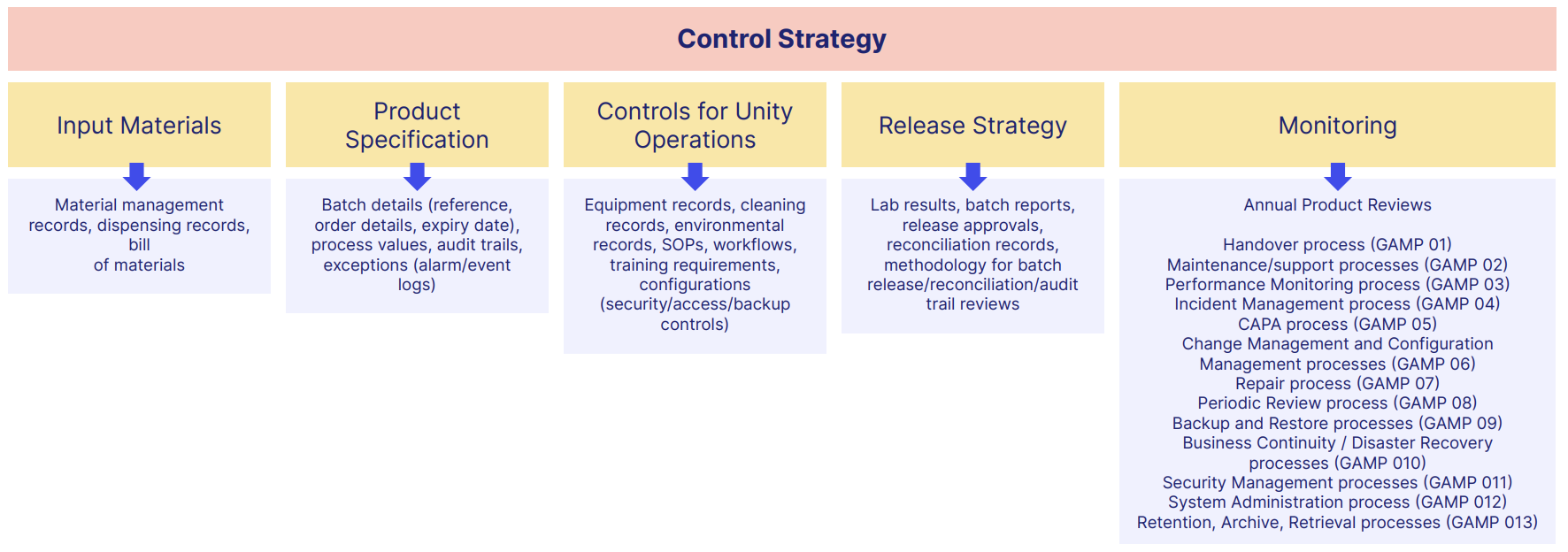
Guidelines for GMP across industries
While the core principles of GMP apply across different sectors, certain industries have additional requirements tailored to their specific products.
Let’s take a look at how GMP applies to pharmaceuticals, medical devices and cosmetics.
GMP and pharma
In the pharmaceutical industry, GMP is a critical component of the regulatory framework. It ensures that medicinal products are consistently produced, controlled and even moved to meet the required quality standards for their intended use. Pharmaceutical GMP focuses heavily on aspects such as product formulation, contamination control, and stability testing.
Pharmaceutical companies must adhere to strict regulations set by bodies like the FDA and EMA - and the scope of compliance can be broader than you might imagine.
As we've seen, GMP regulations cover everything from the cleanliness of the manufacturing environment to the packaging and labeling of medicines - but also extend beyond the immediate manufacturing activity to include storage, movement and even marketing of drugs as well.
For that reason, pharma industry actors like pharmacies and testing labs are just as responsible and accountable for GMP compliance as the actual manufacturers are.
It's therefore important to consider that GMP is a potentially misleading and overly narrow label, and non-compliance can result in severe penalties, including product recalls, fines, and loss of manufacturing, pharmacy or testing accreditation.
Nevertheless, GMP is arguably the single most important area of quality and compliance for drug manufacturers to focus on. GMP non-compliance accounted for 58% of the warning letters issued to US drug manufacturers by the FDA in 2024.
And as you plan your own GMP pharma activity, it can be helpful to know where other businesses typically fall down.
The most common FDA GMP pharma citations are as follows:

And interestingly, data integrity - not often considered a central part of GMP - represents a consistent compliance weakness for pharma manufacturers in the US, referenced in about a fifth of warning letters between 2019 and 2024.

RELATED READING: What is cGMP in the pharma industry?
GMP for medical devices
GMP for medical devices is equally stringent, though the focus is slightly different. Medical devices must not only be safe and effective but also reliable, as they are often used in life-saving situations.
Device manufacturers must ensure that their products meet all regulatory requirements for quality and safety throughout the product's lifecycle.
This includes proper validation of manufacturing processes, equipment calibration, and thorough testing of the devices under various conditions. Manufacturers must also maintain detailed records that trace every stage of production, allowing for quick identification of any issues that may arise after the product is on the market.
Key medical device GMP standards include FDA 21 CFR 820, ISO 13485, and the EU MDR.
GMP for cosmetics
GMP compliance in the cosmetics industry ensures that beauty products are safe for human use.
GMP is mandatory in the EU, but until 2022 was only encouraged in the US. The launch of the FDA's Modernization of Cosmetics Regulation Act (MoCRA) in that year, however, brought the US in line with other areas of the world in mandating GMP for cosmetics manufacturers.
As in pharma and medical device companies, cosmetic GMP guidelines focus on key areas like preventing adulteration, ensuring proper labeling, and maintaining the quality and efficacy of the cosmetic product throughout its shelf life.
Cosmetic manufacturers must also establish systems to control the sourcing of raw materials, particularly ensuring that no harmful chemicals are present. Additionally, cosmetic GMP requires that manufacturers implement proper hygiene practices for workers and ensure that facilities are clean and well-maintained.
The primary international standard for cosmetics GMP is ISO 22716.
GMP compliance
Achieving and maintaining GMP compliance is crucial for manufacturers, as non-compliance can result in legal consequences, product recalls, or even the closure of the facility.
Regulatory bodies around the world oversee GMP compliance, and companies must be prepared for inspections and audits to verify that they meet the required standards.
Regulatory bodies
There are multiple territory-specific regulatory bodies that enforce GMP standards. These agencies have the authority to inspect facilities, review documentation, and issue penalties for non-compliance.
Let's take a look at three key regulatory bodies responsible for overseeing GMP compliance.
FDA
The U.S. Food and Drug Administration (FDA) is one of the most prominent regulatory agencies when it comes to GMP, and its inclusion of the cGMP guide in the Federal Register in 1978 marked the first legally enforceable GMP benchmark.
The FDA oversees GMP compliance for pharmaceuticals, medical devices, food and cosmetics in the United States. The FDA conducts regular inspections of manufacturing facilities to ensure that they meet GMP standards. If violations are found, the FDA can issue warning letters, mandate product recalls, or impose fines.
For FDA GMP compliance, you'll need to comply with 21 CFR Part 210 if you make, process, pack or hold drugs. Even pharmacies - not 'manufacturers' in the true sense of the word - need to meet Part 210 best practice for how their drugs are stored and distributed.
21 CFR Part 211 covers finished pharmaceuticals, while Part 212 is an even more focused standard reserved exclusively for manufacturers of positron emission tomography (PET) drugs such as fluorodeoxyglucose.
GMP for pharma companies under FDA regulation even covers the marketing of the drug, with Part 314 governing these expectations.
And it's also important to note that FDA 21 CFR Part 600 is a key GMP guideline for biotech manufacturers.
EMA
The European Medicines Agency (EMA) is the primary regulatory body overseeing pharmaceutical GMP in Europe.
The EMA works with national agencies in member countries to ensure that medicines are manufactured according to GMP standards. It also inspects facilities outside the EU that export products into Europe, to ensure compliance with EU regulations.
EudraLex is the collection of rules and regulations governing medicinal product in the EU, and its Volume 4 focuses specifically on GMP.
Within Part I of Volume 4, there are 9 GMP chapters (bear with me!) which provide a good indicator of the kind of operational ingredients you'll need to think about for your own GMP compliance.
They are as follows:

WHO
The World Health Organization (WHO) is a global body that provides guidelines for GMP, particularly for medicines and vaccines.
While the WHO itself doesn't regulate or enforce GMP, over 100 countries have adopted its guidelines or used them as the basis for their own national regulations.
The WHO's GMP guidelines are particularly important in developing countries where national regulatory frameworks may be less established, and important work like UN agency vaccine procurement and the WHO Certification Scheme use WHO GMP guidelines as their operating structure.
The WHO began drafting GMP best practice in 1968, and since then its TRS 986 Annex 2 and TRS 999 Annex 2 guidance documents have mapped out GMP benchmarks for pharmaceutical and biotech companies respectively.
The WHO website hosts a range of detailed good manufacturing practices for even more niche subsets, too, such as for investigational products or medicinal gases.
RELATED READING: GxP compliance: processes, challenges and tools
What is GMP certification?
GMP certification, in the sense of being formally designated as GMP-compliant, doesn't really exist.
As we've seen, GMP requirements bleed into other, specific regulatory standards - some of which (like ISO standards) you can be certified to, others you can't.
You could, for instance, get certified to ISO 22716 or ISO 13485, which indirectly demonstrate good manufacturing practice for cosmetic or medical device products.
But you can't get an FDA 21 CFR Part 210 or Part 820 certificate, for instance, or just a plain old 'GMP certificate'.
The reason? You need GMP compliance to manufacture a regulated product in territories like the US. If you're operational and marketing a regulated product, the implication is that you're doing so in a GMP-compliant way. If you weren't, your ability to operate at all would be removed entirely.
A GMP certificate is therefore an unnecessary marker. Some ISO certificates do show you have GMP in place, but are often used to demonstrate other quality and operational elements are in place as well in order to facilitate access to international markets.
There are certain limited cases where a kind of GMP certificate could be issued: if you're exporting a drug overseas, for instance, you can request that your competent authority issue you with a WHO GMP certificate, as the customs authorities of some non-EU countries might request one as proof that your drug has been made and moved in the proper way.
These 'export certificates' are the closest you'll get to a GMP certificate.
Market access is your reward for GMP, not a certificate!
GMP audits
Where do audits fit into all this?
A GMP audit is an in-depth review of your facilities, processes and documentation to ensure compliance with GMP standards. Audits can be conducted by regulatory agencies, third-party auditors or internal quality teams. The audit process typically includes an inspection of the manufacturing environment, a review of documentation, and interviews with employees.
GMP audits are essential for maintaining certification and identifying areas where improvements can be made.
Regular internal audits help manufacturers catch and address compliance issues before they are discovered during an external audit, potentially saving the company from costly fines or production halts.
Benefits of GMP compliance
The benefits of adhering to GMP guidelines extend beyond just regulatory approval. Compliance helps manufacturers ensure product quality, reduce risks, and enhance their market presence.
Below are some key benefits of GMP compliance.
Quality and safety
One of the most significant benefits of GMP compliance is that it ensures the quality and safety of your products. Whether it's a pharmaceutical, medical device or cosmetic product, adhering to GMP standards guarantees that the product meets all necessary specifications and is safe for consumer use.
This reduces the likelihood of defects, recalls, or customer complaints.
Reduced risk
GMP helps reduce risks in the manufacturing process by ensuring that all potential hazards are identified and controlled. These risks could include contamination, incorrect formulations, or equipment failures. By maintaining a controlled and consistent environment, manufacturers can prevent errors that could lead to costly recalls or harm to consumers.
Regulatory approval and market access
Achieving GMP compliance is often a prerequisite for gaining regulatory approval to sell products in many countries. Regulatory bodies, such as the FDA and EMA, require manufacturers to adhere to GMP standards before granting approval to market their products. Without consistent GMP compliance, manufacturers face significant barriers to entry in these markets.
And the average industry costs of, say, pharmaceutical or biotech GMP non-compliance can be significant:
- Cost of a Form 483 observation: $100-200K
- Cost of a warning letter: $50-250M
- Cost of an import alert: $150-500M
- Cost of a consent decree: $2B
- Cost of closing forever: $?
GMP compliance enhances market access by establishing trust with customers, distributors and retailers. Products that are manufactured in accordance with GMP standards are much more likely to be accepted by buyers and health professionals - so treat GMP as an essential, non-negotiable part of how you do business.
Conclusion: embedding GMP at your organization
Good Manufacturing Practice (GMP) is an essential and often mandatory framework that helps you produce safe, high-quality products for your patients and customers.
Understanding and adhering to GMP guidelines is the first step towards regulatory compliance, long-lasting patient trust, and an effective business operation.
But that isn't easy.
More and more life science companies are turning to tools like pharmaceutical quality management software to make GMP a natural, automatic part of their operations.
Functionality like document control, training reminders and configurable event workflows help regulated GMP companies like yours address the 5 Ps, spot and fix areas of non-conformance, and embed lasting GMP and GxP.
Struggling to get consistent GMP in place? Our quality software can help!

